Hyperglycemia Promotes Chemoresistance Through the Reduction of the Mitochondrial DNA Damage, the Bax/Bcl-2 and Bax/Bcl-XL Ratio, and the Cells in Sub-G1 Phase Due to Antitumoral Drugs Induced-Cytotoxicity in Human Colon Adenocarcinoma Cells
- PMID: 30150934
- PMCID: PMC6099160
- DOI: 10.3389/fphar.2018.00866
Hyperglycemia Promotes Chemoresistance Through the Reduction of the Mitochondrial DNA Damage, the Bax/Bcl-2 and Bax/Bcl-XL Ratio, and the Cells in Sub-G1 Phase Due to Antitumoral Drugs Induced-Cytotoxicity in Human Colon Adenocarcinoma Cells
Abstract
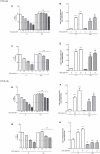
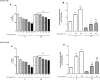
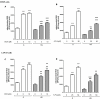
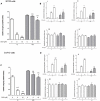
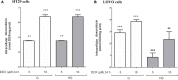
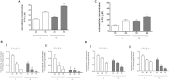
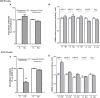
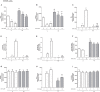
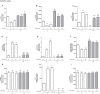
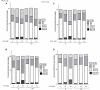
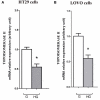
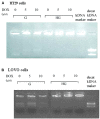
Diabetes and cancer are common, chronic, and potentially fatal diseases that frequently co-exist. Observational studies clearly indicate that the risk of several types of cancer is increased in diabetic patients and a number of cancer types have shown a higher mortality rate in patients with hyperglycemic associated pathologies. This scenario could be due, at least in part, to a lower efficacy of the cancer treatments which needs to be better investigated. Here, we evaluated the effects of a prolonged exposure to high glucose (HG) to the response to chemotherapy on human colon adenocarcinoma HT29 and LOVO cell lines. We observed that hyperglycemia protected against the decreased cell viability and cytotoxicity and preserved from the mitochondrial DNA lesions induced by doxorubicin (DOX) and 5-fluorouracil (5-FU) treatments by lowering ROS production. In HT29 cells the amount of intracellular DOX and its nuclear localization were not modified by HG incubation in terms of Pgp, BCRP, MRP1, 5 and 8 activity and gene expression. On the contrary, in LOVO cells, the amount of intracellular DOX was significantly decreased after a bolus of DOX in HG condition and the expression and activity of MPR1 was increased, suggesting that HG promotes drug chemoresistance in both HT29 and LOVO cells, but in a different way. In both cell types, HG condition prevented the susceptibility to apoptosis by decreasing the ratio Bax/Bcl-2 and Bax/Bcl-XL and diminished the level of cytosolic cytochrome c and the cleavage of full length of PARP induced by DOX and 5-FU. Finally, hyperglycemia reduced cell death by decreasing the cell percentage in sub-G1 peak induced by DOX (via a cell cycle arrest in the G2/M phase) and 5-FU (via a cell cycle arrest in the S phase) in HT29 and LOVO cells. Taken together, our data showed that a prolonged exposure to HG protects human colon adenocarcinoma cells from the cytotoxic effects of two widely used chemotherapeutic drugs, impairing the effectiveness of the chemotherapy itself.
Keywords: 5-fluorouracil; cell damage; chemoresistance; colon adenocarcinoma; cytotoxicity; doxorubicin; hyperglycemia.
Figures


Similar articles
-
The GLP-1R Agonist Exendin-4 Attenuates Hyperglycemia-Induced Chemoresistance in Human Endometrial Cancer Cells Through ROS-Mediated Mitochondrial Pathway.Front Oncol. 2021 Dec 20;11:793530. doi: 10.3389/fonc.2021.793530. eCollection 2021. Front Oncol. 2021. PMID: 34988025 Free PMC article.
-
Inhibition of Pgp activity and cell cycle-dependent chemosensitivity to doxorubicin in the multidrug-resistant LoVo human colon cancer cell line.Eur J Cancer. 1996 Aug;32A(9):1591-7. doi: 10.1016/0959-8049(96)00113-x. Eur J Cancer. 1996. PMID: 8911123
-
High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells.DNA Cell Biol. 2014 Feb;33(2):64-72. doi: 10.1089/dna.2013.2161. Epub 2013 Nov 27. DNA Cell Biol. 2014. PMID: 24283362 Free PMC article.
-
The Bcl-xL and Bax-alpha control points: modulation of apoptosis induced by cancer chemotherapy and relation to TPCK-sensitive protease and caspase activation.Biochem Cell Biol. 1997;75(4):301-14. Biochem Cell Biol. 1997. PMID: 9493953 Review.
-
Hyperglycemia and Chemoresistance in Breast Cancer: From Cellular Mechanisms to Treatment Response.Front Oncol. 2021 Feb 25;11:628359. doi: 10.3389/fonc.2021.628359. eCollection 2021. Front Oncol. 2021. PMID: 33718202 Free PMC article. Review.
Cited by
-
Sanghuangporus sanghuang extract inhibits the proliferation and invasion of lung cancer cells in vitro and in vivo.Nutr Res Pract. 2023 Dec;17(6):1070-1083. doi: 10.4162/nrp.2023.17.6.1070. Epub 2023 Nov 8. Nutr Res Pract. 2023. PMID: 38053828 Free PMC article.
-
Counteracting Action of Curcumin on High Glucose-Induced Chemoresistance in Hepatic Carcinoma Cells.Front Oncol. 2021 Oct 6;11:738961. doi: 10.3389/fonc.2021.738961. eCollection 2021. Front Oncol. 2021. PMID: 34692517 Free PMC article.
-
Type 2 diabetes induced microbiome dysbiosis is associated with therapy resistance in pancreatic adenocarcinoma.Microb Cell Fact. 2020 Mar 24;19(1):75. doi: 10.1186/s12934-020-01330-3. Microb Cell Fact. 2020. PMID: 32204699 Free PMC article.
-
Molecular Mechanism for Selective Cytotoxicity towards Cancer Cells of Diselenide-Containing Paclitaxel Nanoparticles.Int J Biol Sci. 2019 Jul 3;15(8):1755-1770. doi: 10.7150/ijbs.34878. eCollection 2019. Int J Biol Sci. 2019. PMID: 31360117 Free PMC article.
-
Analysis of In Vitro Cyto- and Genotoxicity of Barbatimão Extract on Human Keratinocytes and Fibroblasts.Biomed Res Int. 2018 Oct 8;2018:1942451. doi: 10.1155/2018/1942451. eCollection 2018. Biomed Res Int. 2018. PMID: 30402464 Free PMC article.
References
-
- Atlas Website (2014). Diabetes Research and Clinical Practice and on the IDF Diabetes Atlas Website. Available at: www.idf.org/diabetesatlas
-
- Bergandi L., Aina V., Garetto S., Malavasi G., Aldieri E., Laurenti E., et al. (2010). Fluoride-containing bioactive glasses inhibit pentose phosphate oxidative pathway and glucose 6-phosphate dehydrogenase activity in human osteoblasts. Chem. Biol. Interact. 183 405–415. 10.1016/j.cbi.2009.11.021 - DOI - PubMed
-
- Beutler E. (1971). Red Cell Metabolism A Manual of Biochemical Methods. New York, NY: Grune & Stratton.
-
- Biadgo B., Melku M., Abebe S. M., Abebe M. (2016). Hematological indices and their correlation with fasting blood glucose level and anthropometric measurements in type 2 diabetes mellitus patients in Gondar, Northwest Ethiopia. Diabetes Metab. Syndr. Obes. 9 91–99. 10.2147/DMSO.S97563 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

