Phospholipid synthesis fueled by lipid droplets drives the structural development of poliovirus replication organelles
- PMID: 30148882
- PMCID: PMC6128640
- DOI: 10.1371/journal.ppat.1007280
Phospholipid synthesis fueled by lipid droplets drives the structural development of poliovirus replication organelles
Abstract
Rapid development of complex membranous replication structures is a hallmark of picornavirus infections. However, neither the mechanisms underlying such dramatic reorganization of the cellular membrane architecture, nor the specific role of these membranes in the viral life cycle are sufficiently understood. Here we demonstrate that the cellular enzyme CCTα, responsible for the rate-limiting step in phosphatidylcholine synthesis, translocates from the nuclei to the cytoplasm upon infection and associates with the replication membranes, resulting in the rerouting of lipid synthesis from predominantly neutral lipids to phospholipids. The bulk supply of long chain fatty acids necessary to support the activated phospholipid synthesis in infected cells is provided by the hydrolysis of neutral lipids stored in lipid droplets. Such activation of phospholipid synthesis drives the massive membrane remodeling in infected cells. We also show that complex membranous scaffold of replication organelles is not essential for viral RNA replication but is required for protection of virus propagation from the cellular anti-viral response, especially during multi-cycle replication conditions. Inhibition of infection-specific phospholipid synthesis provides a new paradigm for controlling infection not by suppressing viral replication but by making it more visible to the immune system.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
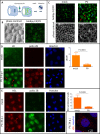
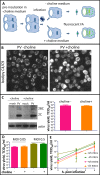
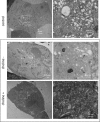
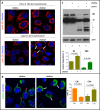
Similar articles
-
Increased long chain acyl-Coa synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles.PLoS Pathog. 2013;9(6):e1003401. doi: 10.1371/journal.ppat.1003401. Epub 2013 Jun 6. PLoS Pathog. 2013. PMID: 23762027 Free PMC article.
-
Fluorescent fatty acid analogs as a tool to study development of the picornavirus replication organelles.J Virol Methods. 2014 May;200:15-21. doi: 10.1016/j.jviromet.2014.01.020. Epub 2014 Feb 3. J Virol Methods. 2014. PMID: 24503038
-
The Role of Electron Microscopy in Studying the Continuum of Changes in Membranous Structures during Poliovirus Infection.Viruses. 2015 Oct 12;7(10):5305-18. doi: 10.3390/v7102874. Viruses. 2015. PMID: 26473912 Free PMC article. Review.
-
Complex lipid metabolic remodeling is required for efficient hepatitis C virus replication.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Sep;1863(9):1041-1056. doi: 10.1016/j.bbalip.2018.06.002. Epub 2018 Jun 6. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29885363
-
Involvement of cellular membrane traffic proteins in poliovirus replication.Cell Cycle. 2007 Jan 1;6(1):36-8. doi: 10.4161/cc.6.1.3683. Epub 2007 Jan 11. Cell Cycle. 2007. PMID: 17245115 Review.
Cited by
-
Enterovirus Infection Induces Massive Recruitment of All Isoforms of Small Cellular Arf GTPases to the Replication Organelles.J Virol. 2020 Dec 22;95(2):e01629-20. doi: 10.1128/JVI.01629-20. Print 2020 Dec 22. J Virol. 2020. PMID: 33087467 Free PMC article.
-
Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses.Viruses. 2020 Jun 24;12(6):682. doi: 10.3390/v12060682. Viruses. 2020. PMID: 32599855 Free PMC article. Review.
-
Lipid Droplets: Formation, Degradation, and Their Role in Cellular Responses to Flavivirus Infections.Microorganisms. 2024 Mar 24;12(4):647. doi: 10.3390/microorganisms12040647. Microorganisms. 2024. PMID: 38674592 Free PMC article. Review.
-
Identification and Characterization of Human Norovirus NTPase Regions Required for Lipid Droplet Localization, Cellular Apoptosis, and Interaction with the Viral P22 Protein.Microbiol Spectr. 2021 Sep 3;9(1):e0042221. doi: 10.1128/Spectrum.00422-21. Epub 2021 Aug 25. Microbiol Spectr. 2021. PMID: 34431704 Free PMC article.
-
Lipid compartments and lipid metabolism as therapeutic targets against coronavirus.Front Immunol. 2023 Dec 1;14:1268854. doi: 10.3389/fimmu.2023.1268854. eCollection 2023. Front Immunol. 2023. PMID: 38106410 Free PMC article. Review.
References
-
- Knowlton KU. CVB infection and mechanisms of viral cardiomyopathy. Curr Top Microbiol Immunol. 2008;323:315–35. Epub 2008/03/25. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

