Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating
- PMID: 30139744
- PMCID: PMC6187627
- DOI: 10.1074/jbc.RA118.005066
Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating
Abstract
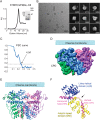
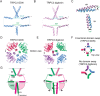
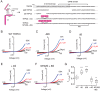
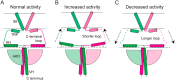
The transient receptor potential ion channels support Ca2+ permeation in many organs, including the heart, brain, and kidney. Genetic mutations in transient receptor potential cation channel subfamily C member 3 (TRPC3) are associated with neurodegenerative diseases, memory loss, and hypertension. To better understand the conformational changes that regulate TRPC3 function, we solved the cryo-EM structures for the full-length human TRPC3 and its cytoplasmic domain (CPD) in the apo state at 5.8- and 4.0-Å resolution, respectively. These structures revealed that the TRPC3 transmembrane domain resembles those of other TRP channels and that the CPD is a stable module involved in channel assembly and gating. We observed the presence of a C-terminal domain swap at the center of the CPD where horizontal helices (HHs) transition into a coiled-coil bundle. Comparison of TRPC3 structures revealed that the HHs can reside in two distinct positions. Electrophysiological analyses disclosed that shortening the length of the C-terminal loop connecting the HH with the TRP helices increases TRPC3 activity and that elongating the length of the loop has the opposite effect. Our findings indicate that the C-terminal loop affects channel gating by altering the allosteric coupling between the cytoplasmic and transmembrane domains. We propose that molecules that target the HH may represent a promising strategy for controlling TRPC3-associated neurological disorders and hypertension.
Keywords: GSK-1702934A; TRPC3; calcium channel; cryo-electron microscopy; electrophysiology; ion channel; neurotransmitter; structural biology; transient receptor potential channels (TRP channels); vascular biology.
© 2018 Sierra-Valdez et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Cryo-EM structure of the cytoplasmic domain of murine transient receptor potential cation channel subfamily C member 6 (TRPC6).J Biol Chem. 2018 Jun 29;293(26):10381-10391. doi: 10.1074/jbc.RA118.003183. Epub 2018 May 11. J Biol Chem. 2018. PMID: 29752403 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
Hydrophobic interactions within the C terminus pole helices tunnel regulate calcium-dependent inactivation of TRPC3 in a calmodulin-dependent manner.Cell Calcium. 2023 Jan;109:102684. doi: 10.1016/j.ceca.2022.102684. Epub 2022 Nov 30. Cell Calcium. 2023. PMID: 36495796 Free PMC article.
-
Structure of the receptor-activated human TRPC6 and TRPC3 ion channels.Cell Res. 2018 Jul;28(7):746-755. doi: 10.1038/s41422-018-0038-2. Epub 2018 Apr 26. Cell Res. 2018. PMID: 29700422 Free PMC article.
Cited by
-
Heterogeneity in Slow Synaptic Transmission Diversifies Purkinje Cell Timing.J Neurosci. 2024 Sep 11;44(37):e0455242024. doi: 10.1523/JNEUROSCI.0455-24.2024. J Neurosci. 2024. PMID: 39147589 Free PMC article.
-
Transient Receptor Potential Canonical (TRPC) Channels: Then and Now.Cells. 2020 Aug 28;9(9):1983. doi: 10.3390/cells9091983. Cells. 2020. PMID: 32872338 Free PMC article. Review.
-
TRPC channels: Structure, function, regulation and recent advances in small molecular probes.Pharmacol Ther. 2020 May;209:107497. doi: 10.1016/j.pharmthera.2020.107497. Epub 2020 Jan 28. Pharmacol Ther. 2020. PMID: 32004513 Free PMC article. Review.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
-
Transient Receptor Potential Canonical 6 (TRPC6) Channel in the Pathogenesis of Diseases: A Jack of Many Trades.Inflammation. 2023 Aug;46(4):1144-1160. doi: 10.1007/s10753-023-01808-3. Epub 2023 Apr 18. Inflammation. 2023. PMID: 37072606 Free PMC article. Review.
References
-
- Bröker-Lai J., Kollewe A., Schindeldecker B., Pohle J., Nguyen Chi V., Mathar I., Guzman R., Schwarz Y., Lai A., Weißgerber P., Schwegler H., Dietrich A., Both M., Sprengel R., Draguhn A., et al. (2017) Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory. EMBO J. 36, 2770–2789 10.15252/embj.201696369 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

