Identification and characterization of ubiquitinylation sites in TAR DNA-binding protein of 43 kDa (TDP-43)
- PMID: 30120199
- PMCID: PMC6187624
- DOI: 10.1074/jbc.RA118.003440
Identification and characterization of ubiquitinylation sites in TAR DNA-binding protein of 43 kDa (TDP-43)
Abstract
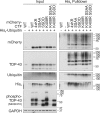
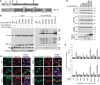
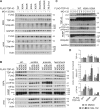
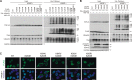
TAR DNA-binding protein of 43 kDa (TDP-43) forms pathological aggregates in neurodegenerative diseases, particularly in certain forms of frontotemporal dementia and amyotrophic lateral sclerosis. Pathological modifications of TDP-43 include proteolytic fragmentation, phosphorylation, and ubiquitinylation. A pathognomonic TDP-43 C-terminal fragment (CTF) spanning amino acids 193-414 contains only four lysine residues that could be potentially ubiquitinylated. Here, serial mutagenesis of these four lysines to arginine revealed that not a single residue is responsible for the ubiquitinylation of mCherry-tagged CTF. Removal of all four lysines was necessary to suppress ubiquitinylation. Interestingly, Lys-408 substitution enhanced the pathological phosphorylation of the immediately adjacent serine residues 409/410 in the context of mCherry-CTF. Thus, Lys-408 ubiquitinylation appears to hinder Ser-409/410 phosphorylation in TDP-43 CTF. However, we did not observe the same effect for full-length TDP-43. We extended the mutagenesis study to full-length TDP-43 and performed MS. Ubiquitinylated lysine residues were identified in the nuclear localization sequence (NLS; Lys-84 and Lys-95) and RNA-binding region (mostly Lys-160, Lys-181, and Lys-263). Mutagenesis of Lys-84 confirmed its importance as the major determinant for nuclear import, whereas Lys-95 mutagenesis did not significantly affect TDP-43's nucleo-cytoplasmic distribution, solubility, aggregation, and RNA-processing activities. Nevertheless, the K95A mutant had significantly reduced Ser-409/410 phosphorylation, emphasizing the suspected interplay between TDP-43 ubiquitinylation and phosphorylation. Collectively, our analysis of TDP-43 ubiquitinylation sites indicates that the NLS residues Lys-84 and Lys-95 have more prominent roles in TDP-43 function than the more C-terminal lysines and suggests a link between specific ubiquitinylation events and pathological TDP-43 phosphorylation.
Keywords: TDP-43; amyotrophic lateral sclerosis (ALS) (Lou Gehrig disease); frontotemporal dementia; mass spectrometry (MS); neurodegeneration; proteasome; protein aggregation; protein phosphorylation; site-directed mutagenesis; ubiquitylation (ubiquitination).
© 2018 Hans et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Point mutations in the N-terminal domain of transactive response DNA-binding protein 43 kDa (TDP-43) compromise its stability, dimerization, and functions.J Biol Chem. 2017 Jul 14;292(28):11992-12006. doi: 10.1074/jbc.M117.775965. Epub 2017 May 31. J Biol Chem. 2017. PMID: 28566288 Free PMC article.
-
Detection of TAR DNA-binding protein 43 (TDP-43) oligomers as initial intermediate species during aggregate formation.J Biol Chem. 2019 Apr 26;294(17):6696-6709. doi: 10.1074/jbc.RA118.005889. Epub 2019 Mar 1. J Biol Chem. 2019. PMID: 30824544 Free PMC article.
-
Multiple distinct pathways lead to hyperubiquitylated insoluble TDP-43 protein independent of its translocation into stress granules.J Biol Chem. 2020 Jan 17;295(3):673-689. doi: 10.1074/jbc.RA119.010617. Epub 2019 Nov 28. J Biol Chem. 2020. PMID: 31780563 Free PMC article.
-
[Neurodegenerative disorders and TDP-43].Brain Nerve. 2009 Feb;61(2):161-6. Brain Nerve. 2009. PMID: 19235466 Review. Japanese.
-
[TDP-43 proteinopathies, toward understanding of the molecular pathogenesis].Rinsho Shinkeigaku. 2009 Nov;49(11):783-5. doi: 10.5692/clinicalneurol.49.783. Rinsho Shinkeigaku. 2009. PMID: 20030209 Review. Japanese.
Cited by
-
Suppression of Linear Ubiquitination Ameliorates Cytoplasmic Aggregation of Truncated TDP-43.Cells. 2022 Aug 3;11(15):2398. doi: 10.3390/cells11152398. Cells. 2022. PMID: 35954242 Free PMC article.
-
Stress granule subtypes: an emerging link to neurodegeneration.Cell Mol Life Sci. 2020 Dec;77(23):4827-4845. doi: 10.1007/s00018-020-03565-0. Epub 2020 Jun 4. Cell Mol Life Sci. 2020. PMID: 32500266 Free PMC article. Review.
-
Sirtuin-1 sensitive lysine-136 acetylation drives phase separation and pathological aggregation of TDP-43.Nat Commun. 2022 Mar 9;13(1):1223. doi: 10.1038/s41467-022-28822-7. Nat Commun. 2022. PMID: 35264561 Free PMC article.
-
Proteomics Approaches for Biomarker and Drug Target Discovery in ALS and FTD.Front Neurosci. 2019 Jun 11;13:548. doi: 10.3389/fnins.2019.00548. eCollection 2019. Front Neurosci. 2019. PMID: 31244593 Free PMC article. Review.
-
The pathogenic mechanism of TAR DNA-binding protein 43 (TDP-43) in amyotrophic lateral sclerosis.Neural Regen Res. 2024 Apr;19(4):800-806. doi: 10.4103/1673-5374.382233. Neural Regen Res. 2024. PMID: 37843214 Free PMC article. Review.
References
-
- Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., et al. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 10.1126/science.1134108 - DOI - PubMed
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., and Oda T. (2006) TDP-43 is a component of ubiquitin-positive τ-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 10.1016/j.bbrc.2006.10.093 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

