Immunogenicity and Protection After Vaccination With a Modified Vaccinia Virus Ankara-Vectored Yellow Fever Vaccine in the Hamster Model
- PMID: 30116244
- PMCID: PMC6082969
- DOI: 10.3389/fimmu.2018.01756
Immunogenicity and Protection After Vaccination With a Modified Vaccinia Virus Ankara-Vectored Yellow Fever Vaccine in the Hamster Model
Abstract
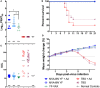
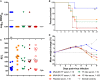
The highly efficacious live-attenuated 17D yellow fever (YF) vaccine is occasionally associated with rare life-threatening adverse events. Modified vaccinia virus Ankara (MVA), a non-replicating poxvirus, has been used as a vaccine platform to safely deliver various antigens. A MVA-based YF vaccine (MVA-BN-YF) was tested with and without a non-mineral oil adjuvant in a hamster model of lethal YF disease and protective efficacy of this vaccine was compared with the 17D vaccine. The vaccine candidate MVA-BN-YF generated a protective response in hamsters infected with YFV that was comparable to protection by the live 17D vaccine. Similar levels of neutralizing antibody were observed in animals vaccinated with either vaccine alone or vaccine with adjuvant. Significant improvement in survival, weight change, and serum alanine aminotransferase levels were observed in vaccinated hamsters when administered 42 and 14 days prior to challenge with Jimenez YF virus (YFV). Neutralizing antibodies induced by MVA-BN-YF were transferred to naïve hamsters prior to virus challenge. Passive administration of neutralizing antibody 24 h prior to virus infection resulted in significantly improved survival and weight change. A trend toward reduced liver enzyme levels was also observed. MVA-BN-YF, therefore, represents a safe alternative to vaccination with live-attenuated YFV.
Keywords: 17D; hamster; modified vaccinia virus Ankara; neutralizing antibodies; passive immunization; yellow fever.
Figures
Similar articles
-
Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model.Vaccine. 2011 Aug 11;29(35):6008-16. doi: 10.1016/j.vaccine.2011.06.034. Epub 2011 Jun 28. Vaccine. 2011. PMID: 21718741 Free PMC article.
-
Molecular and immunological characterization of a DNA-launched yellow fever virus 17D infectious clone.J Gen Virol. 2015 Apr;96(Pt 4):804-814. doi: 10.1099/jgv.0.000026. Epub 2014 Dec 16. J Gen Virol. 2015. PMID: 25516543 Free PMC article.
-
Development of a Bicistronic Yellow Fever Live Attenuated Vaccine with Reduced Neurovirulence and Viscerotropism.Microbiol Spectr. 2022 Oct 26;10(5):e0224622. doi: 10.1128/spectrum.02246-22. Epub 2022 Aug 18. Microbiol Spectr. 2022. PMID: 35980184 Free PMC article.
-
Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease.Vaccine. 2017 Oct 20;35(44):5951-5955. doi: 10.1016/j.vaccine.2017.03.032. Epub 2017 Mar 30. Vaccine. 2017. PMID: 28366605 Review.
-
Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place?Curr Opin Immunol. 2009 Jun;21(3):308-13. doi: 10.1016/j.coi.2009.05.018. Epub 2009 Jun 10. Curr Opin Immunol. 2009. PMID: 19520559 Review.
Cited by
-
The Present and Future of Yellow Fever Vaccines.Pharmaceuticals (Basel). 2021 Sep 1;14(9):891. doi: 10.3390/ph14090891. Pharmaceuticals (Basel). 2021. PMID: 34577591 Free PMC article. Review.
-
A Yellow Fever Virus 17D Infection and Disease Mouse Model Used to Evaluate a Chimeric Binjari-Yellow Fever Virus Vaccine.Vaccines (Basel). 2020 Jul 9;8(3):368. doi: 10.3390/vaccines8030368. Vaccines (Basel). 2020. PMID: 32660106 Free PMC article.
-
Efficacy of H2O2 inactivated bovine virus diarrhoea virus (BVDV) type 1 vaccine in mice.BMC Vet Res. 2024 Feb 3;20(1):43. doi: 10.1186/s12917-024-03897-0. BMC Vet Res. 2024. PMID: 38308297 Free PMC article.
-
Disease Resurgence, Production Capability Issues and Safety Concerns in the Context of an Aging Population: Is There a Need for a New Yellow Fever Vaccine?Vaccines (Basel). 2019 Nov 8;7(4):179. doi: 10.3390/vaccines7040179. Vaccines (Basel). 2019. PMID: 31717289 Free PMC article. Review.
-
Rendezvous with Vaccinia Virus in the Post-smallpox Era: R&D Advances.Viruses. 2023 Aug 15;15(8):1742. doi: 10.3390/v15081742. Viruses. 2023. PMID: 37632084 Free PMC article. Review.
References
-
- Barrett AD, Monath TP, Barban V, Niedrig M, Teuwen DE. 17D yellow fever vaccines: new insights. A report of a workshop held during the World Congress on medicine and health in the tropics, Marseille, France, Monday 12 September 2005. Vaccine (2007) 25:2758–65.10.1016/j.vaccine.2006.12.015 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

