The Methylation Status of the Epigenome: Its Emerging Role in the Regulation of Tumor Angiogenesis and Tumor Growth, and Potential for Drug Targeting
- PMID: 30103412
- PMCID: PMC6115976
- DOI: 10.3390/cancers10080268
The Methylation Status of the Epigenome: Its Emerging Role in the Regulation of Tumor Angiogenesis and Tumor Growth, and Potential for Drug Targeting
Abstract
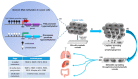
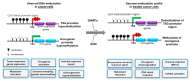
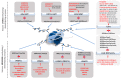
Approximately 50 years ago, Judah Folkman raised the concept of inhibiting tumor angiogenesis for treating solid tumors. The development of anti-angiogenic drugs would decrease or even arrest tumor growth by restricting the delivery of oxygen and nutrient supplies, while at the same time display minimal toxic side effects to healthy tissues. Bevacizumab (Avastin)-a humanized monoclonal anti VEGF-A antibody-is now used as anti-angiogenic drug in several forms of cancers, yet with variable results. Recent years brought significant progresses in our understanding of the role of chromatin remodeling and epigenetic mechanisms in the regulation of angiogenesis and tumorigenesis. Many inhibitors of DNA methylation as well as of histone methylation, have been successfully tested in preclinical studies and some are currently undergoing evaluation in phase I, II or III clinical trials, either as cytostatic molecules-reducing the proliferation of cancerous cells-or as tumor angiogenesis inhibitors. In this review, we will focus on the methylation status of the vascular epigenome, based on the genomic DNA methylation patterns with DNA methylation being mainly transcriptionally repressive, and lysine/arginine histone post-translational modifications which either promote or repress the chromatin transcriptional state. Finally, we discuss the potential use of "epidrugs" in efficient control of tumor growth and tumor angiogenesis.
Keywords: DNA methylation; histone methylation; metastasis; tumor angiogenesis.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

Similar articles
-
Epigenetics and Control of Tumor Angiogenesis in Melanoma: An Update with Therapeutic Implications.Cancers (Basel). 2024 Aug 14;16(16):2843. doi: 10.3390/cancers16162843. Cancers (Basel). 2024. PMID: 39199614 Free PMC article. Review.
-
New drugs in cancer therapy, National Tumor Institute, Naples, 17-18 June 2004.Anticancer Drugs. 2005 Feb;16(2):211-21. doi: 10.1097/00001813-200502000-00014. Anticancer Drugs. 2005. PMID: 15655420
-
Tumor angiogenesis: past, present and the near future.Carcinogenesis. 2000 Mar;21(3):505-15. doi: 10.1093/carcin/21.3.505. Carcinogenesis. 2000. PMID: 10688871 Review.
-
Anti-angiogenic therapies in cancer: achievements and open questions.Bull Cancer. 2007 Sep;94(9):753-62. Bull Cancer. 2007. PMID: 17878094 Review.
-
VEGF and colon cancer growth beyond angiogenesis: does VEGF directly mediate colon cancer growth via a non-angiogenic mechanism?Curr Pharm Des. 2014;20(7):1041-4. doi: 10.2174/1381612819999131218175905. Curr Pharm Des. 2014. PMID: 23755727 Review.
Cited by
-
Epigenetics and Control of Tumor Angiogenesis in Melanoma: An Update with Therapeutic Implications.Cancers (Basel). 2024 Aug 14;16(16):2843. doi: 10.3390/cancers16162843. Cancers (Basel). 2024. PMID: 39199614 Free PMC article. Review.
-
Computational methods and next-generation sequencing approaches to analyze epigenetics data: Profiling of methods and applications.Methods. 2021 Mar;187:92-103. doi: 10.1016/j.ymeth.2020.09.008. Epub 2020 Sep 14. Methods. 2021. PMID: 32941995 Free PMC article. Review.
-
Small-Molecule Inhibitors Overcome Epigenetic Reprogramming for Cancer Therapy.Front Pharmacol. 2021 Sep 17;12:702360. doi: 10.3389/fphar.2021.702360. eCollection 2021. Front Pharmacol. 2021. PMID: 34603017 Free PMC article. Review.
-
Antitumor Effect of Demethylzeylasteral (T-96) on Triple-Negative Breast Cancer via LSD1-Mediate Epigenetic Mechanisms.Anal Cell Pathol (Amst). 2022 Oct 12;2022:2522597. doi: 10.1155/2022/2522597. eCollection 2022. Anal Cell Pathol (Amst). 2022. PMID: 36276611 Free PMC article.
-
Histone methylation and vascular biology.Clin Epigenetics. 2020 Feb 18;12(1):30. doi: 10.1186/s13148-020-00826-4. Clin Epigenetics. 2020. PMID: 32070413 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

