Protective Effect of Sex Hormone-Binding Globulin against Metabolic Syndrome: In Vitro Evidence Showing Anti-Inflammatory and Lipolytic Effects on Adipocytes and Macrophages
- PMID: 30046278
- PMCID: PMC6036814
- DOI: 10.1155/2018/3062319
Protective Effect of Sex Hormone-Binding Globulin against Metabolic Syndrome: In Vitro Evidence Showing Anti-Inflammatory and Lipolytic Effects on Adipocytes and Macrophages
Abstract
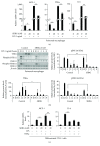
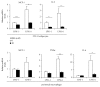
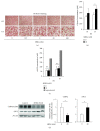
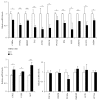
Sex hormone-binding globulin (SHBG) is a serum protein released mainly by the liver, and a low serum level correlates with a risk for metabolic syndrome including diabetes, obesity, and cardiovascular events. However, the underlying molecular mechanism(s) linking SHBG and metabolic syndrome remains unknown. In this study, using adipocytes and macrophages, we focused on the in vitro effects of SHBG on inflammation as well as lipid metabolism. Incubation with 20 nM SHBG markedly suppressed lipopolysaccharide- (LPS-) induced inflammatory cytokines, such as MCP-1, TNFα, and IL-6 in adipocytes and macrophages, along with phosphorylations of JNK and ERK. Anti-inflammatory effects were also observed in 3T3-L1 adipocytes cocultured with LPS-stimulated macrophages. In addition, SHBG treatment for 18 hrs or longer significantly induced the lipid degradation of differentiated 3T3-L1 cells, with alterations in its corresponding gene and protein levels. Notably, these effects of SHBG were not altered by coaddition of large amounts of testosterone or estradiol. In conclusion, SHBG suppresses inflammation and lipid accumulation in macrophages and adipocytes, which might be among the mechanisms underlying the protective effect of SHBG, that is, its actions which reduce the incidence of metabolic syndrome.
Figures
Similar articles
-
Phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3T3-L1 cells and in macrophage-adipocyte co-cultures.Mol Nutr Food Res. 2013 Oct;57(10):1803-13. doi: 10.1002/mnfr.201300001. Epub 2013 Jun 17. Mol Nutr Food Res. 2013. PMID: 23776070
-
Inhibition of adipocyte inflammation and macrophage chemotaxis by butein.Eur J Pharmacol. 2014 Sep 5;738:40-8. doi: 10.1016/j.ejphar.2014.05.031. Epub 2014 May 27. Eur J Pharmacol. 2014. PMID: 24877688
-
Sparstolonin B inhibits lipopolysaccharide-induced inflammation in 3T3-L1 adipocytes.Eur J Pharmacol. 2015 Dec 15;769:79-85. doi: 10.1016/j.ejphar.2015.10.050. Epub 2015 Oct 30. Eur J Pharmacol. 2015. PMID: 26522926
-
AIMing at metabolic syndrome. -Towards the development of novel therapies for metabolic diseases via apoptosis inhibitor of macrophage (AIM).-.Circ J. 2011;75(11):2522-31. doi: 10.1253/circj.cj-11-0891. Epub 2011 Oct 5. Circ J. 2011. PMID: 21970839 Review.
-
Life style-related diseases of the digestive system: endocrine disruptors stimulate lipid accumulation in target cells related to metabolic syndrome.J Pharmacol Sci. 2007 Oct;105(2):133-7. doi: 10.1254/jphs.fm0070034. Epub 2007 Oct 6. J Pharmacol Sci. 2007. PMID: 17928741 Review.
Cited by
-
Sex Hormones and Diabetes in 45- to 74-year-old Men and Postmenopausal Women: The Hispanic Community Health Study.J Clin Endocrinol Metab. 2023 Jun 16;108(7):1709-1726. doi: 10.1210/clinem/dgad018. J Clin Endocrinol Metab. 2023. PMID: 36633580 Free PMC article.
-
Assessment of common risk factors of diabetes and chronic kidney disease: a Mendelian randomization study.Front Endocrinol (Lausanne). 2023 Sep 13;14:1265719. doi: 10.3389/fendo.2023.1265719. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37780623 Free PMC article.
-
Associations Between Dietary Inflammatory Index and Sex Hormones Among 6- to 19-Year-Old Children and Adolescents in NHANES 2015-2016.Front Endocrinol (Lausanne). 2022 Jan 10;12:792114. doi: 10.3389/fendo.2021.792114. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35082755 Free PMC article.
-
Longitudinal associations between sex hormone-binding globulin and insulin resistance.Endocr Connect. 2020 May;9(5):418-425. doi: 10.1530/EC-20-0141. Endocr Connect. 2020. PMID: 32427568 Free PMC article.
-
Sex hormone binding globulin (SHBG) modulates mitochondrial dynamics in PPARγ-depleted equine adipose derived stromal cells.J Mol Med (Berl). 2024 Aug;102(8):1015-1036. doi: 10.1007/s00109-024-02459-z. Epub 2024 Jun 14. J Mol Med (Berl). 2024. PMID: 38874666 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

