Adaptation and Therapeutic Exploitation of the Plasma Membrane of African Trypanosomes
- PMID: 30037058
- PMCID: PMC6071061
- DOI: 10.3390/genes9070368
Adaptation and Therapeutic Exploitation of the Plasma Membrane of African Trypanosomes
Abstract
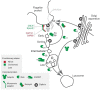
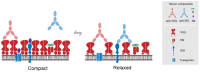
African trypanosomes are highly divergent from their metazoan hosts, and as part of adaptation to a parasitic life style have developed a unique endomembrane system. The key virulence mechanism of many pathogens is successful immune evasion, to enable survival within a host, a feature that requires both genetic events and membrane transport mechanisms in African trypanosomes. Intracellular trafficking not only plays a role in immune evasion, but also in homeostasis of intracellular and extracellular compartments and interactions with the environment. Significantly, historical and recent work has unraveled some of the connections between these processes and highlighted how immune evasion mechanisms that are associated with adaptations to membrane trafficking may have, paradoxically, provided specific sensitivity to drugs. Here, we explore these advances in understanding the membrane composition of the trypanosome plasma membrane and organelles and provide a perspective for how transport could be exploited for therapeutic purposes.
Keywords: Trypanosoma brucei; drug development; endocytosis; endomembrane system; nanobodies; surface proteome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Macromolecular trafficking and immune evasion in african trypanosomes.Int Rev Cell Mol Biol. 2009;278:1-67. doi: 10.1016/S1937-6448(09)78001-3. Int Rev Cell Mol Biol. 2009. PMID: 19815176 Review.
-
The Trypanosome Exocyst: A Conserved Structure Revealing a New Role in Endocytosis.PLoS Pathog. 2017 Jan 23;13(1):e1006063. doi: 10.1371/journal.ppat.1006063. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28114397 Free PMC article.
-
Evidence for recycling of invariant surface transmembrane domain proteins in African trypanosomes.Eukaryot Cell. 2013 Feb;12(2):330-42. doi: 10.1128/EC.00273-12. Epub 2012 Dec 21. Eukaryot Cell. 2013. PMID: 23264644 Free PMC article.
-
Specializations in a successful parasite: what makes the bloodstream-form African trypanosome so deadly?Mol Biochem Parasitol. 2011 Oct;179(2):51-8. doi: 10.1016/j.molbiopara.2011.06.006. Epub 2011 Jul 7. Mol Biochem Parasitol. 2011. PMID: 21763356 Review.
-
African trypanosomes: the genome and adaptations for immune evasion.Essays Biochem. 2011;51:47-62. doi: 10.1042/bse0510047. Essays Biochem. 2011. PMID: 22023441 Review.
Cited by
-
Drug resistance in animal trypanosomiases: Epidemiology, mechanisms and control strategies.Int J Parasitol Drugs Drug Resist. 2024 Aug;25:100533. doi: 10.1016/j.ijpddr.2024.100533. Epub 2024 Mar 30. Int J Parasitol Drugs Drug Resist. 2024. PMID: 38555795 Free PMC article. Review.
-
Therapeutic efficacy of β-sitosterol treatment on Trypanosoma congolense infection, anemia development, and trans-sialidase (TconTS1) gene expression.Front Microbiol. 2023 Sep 27;14:1282257. doi: 10.3389/fmicb.2023.1282257. eCollection 2023. Front Microbiol. 2023. PMID: 37886075 Free PMC article.
-
Black-necked spitting cobra (Naja nigricollis) phospholipases A2 may cause Trypanosoma brucei death by blocking endocytosis through the flagellar pocket.Sci Rep. 2022 Apr 16;12(1):6394. doi: 10.1038/s41598-022-10091-5. Sci Rep. 2022. PMID: 35430620 Free PMC article.
-
Physiological and proteomic profiles of Trypanosoma brucei rhodesiense parasite isolated from suramin responsive and non-responsive HAT patients in Busoga, Uganda.Int J Parasitol Drugs Drug Resist. 2021 Apr;15:57-67. doi: 10.1016/j.ijpddr.2021.02.001. Epub 2021 Feb 9. Int J Parasitol Drugs Drug Resist. 2021. PMID: 33588295 Free PMC article.
-
Instability of aquaglyceroporin (AQP) 2 contributes to drug resistance in Trypanosoma brucei.PLoS Negl Trop Dis. 2020 Jul 9;14(7):e0008458. doi: 10.1371/journal.pntd.0008458. eCollection 2020 Jul. PLoS Negl Trop Dis. 2020. PMID: 32644992 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

