Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC)
- PMID: 29982279
- PMCID: PMC6096736
- DOI: 10.1093/annonc/mdy218
Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC)
Abstract
Background: Neoadjuvant anti-PD-1 may improve outcomes for patients with resectable NSCLC and provides a critical window for examining pathologic features associated with response. Resections showing major pathologic response to neoadjuvant therapy, defined as ≤10% residual viable tumor (RVT), may predict improved long-term patient outcome. However, %RVT calculations were developed in the context of chemotherapy (%cRVT). An immune-related %RVT (%irRVT) has yet to be developed.
Patients and methods: The first trial of neoadjuvant anti-PD-1 (nivolumab, NCT02259621) was just reported. We analyzed hematoxylin and eosin-stained slides from the post-treatment resection specimens of the 20 patients with non-small-cell lung carcinoma who underwent definitive surgery. Pretreatment tumor biopsies and preresection radiographic 'tumor' measurements were also assessed.
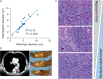
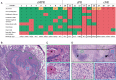
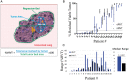
Results: We found that the regression bed (the area of immune-mediated tumor clearance) accounts for the previously noted discrepancy between CT imaging and pathologic assessment of residual tumor. The regression bed is characterized by (i) immune activation-dense tumor infiltrating lymphocytes with macrophages and tertiary lymphoid structures; (ii) massive tumor cell death-cholesterol clefts; and (iii) tissue repair-neovascularization and proliferative fibrosis (each feature enriched in major pathologic responders versus nonresponders, P < 0.05). This distinct constellation of histologic findings was not identified in any pretreatment specimens. Histopathologic features of the regression bed were used to develop 'Immune-Related Pathologic Response Criteria' (irPRC), and these criteria were shown to be reproducible amongst pathologists. Specifically, %irRVT had improved interobserver consistency compared with %cRVT [median per-case %RVT variability 5% (0%-29%) versus 10% (0%-58%), P = 0.007] and a twofold decrease in median standard deviation across pathologists within a sample (4.6 versus 2.2, P = 0.002).
Conclusions: irPRC may be used to standardize pathologic assessment of immunotherapeutic efficacy. Long-term follow-up is needed to determine irPRC reliability as a surrogate for recurrence-free and overall survival.
Figures

Comment in
-
Understanding patterns of pathologic response following neoadjuvant immunotherapy for solid tumors.Ann Oncol. 2018 Aug 1;29(8):1630-1632. doi: 10.1093/annonc/mdy227. Ann Oncol. 2018. PMID: 30052725 No abstract available.
-
Neoadjuvant immunotherapy in non-small cell lung cancer: the sooner the better?Transl Lung Cancer Res. 2018 Dec;7(Suppl 4):S356-S357. doi: 10.21037/tlcr.2018.10.12. Transl Lung Cancer Res. 2018. PMID: 30705854 Free PMC article. No abstract available.
Similar articles
-
Pharmacodynamics of Pre-Operative PD1 checkpoint blockade and receptor activator of NFkB ligand (RANKL) inhibition in non-small cell lung cancer (NSCLC): study protocol for a multicentre, open-label, phase 1B/2, translational trial (POPCORN).Trials. 2019 Dec 19;20(1):753. doi: 10.1186/s13063-019-3951-x. Trials. 2019. PMID: 31856909 Free PMC article.
-
Major pathologic response on biopsy (MPRbx) in patients with advanced melanoma treated with anti-PD-1: evidence for an early, on-therapy biomarker of response.Ann Oncol. 2019 Apr 1;30(4):589-596. doi: 10.1093/annonc/mdz019. Ann Oncol. 2019. PMID: 30689736 Free PMC article. Clinical Trial.
-
Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial.Lancet Oncol. 2020 Jun;21(6):786-795. doi: 10.1016/S1470-2045(20)30140-6. Epub 2020 May 7. Lancet Oncol. 2020. PMID: 32386568 Clinical Trial.
-
Current status of immune checkpoint inhibition in early-stage NSCLC.Ann Oncol. 2019 Aug 1;30(8):1244-1253. doi: 10.1093/annonc/mdz175. Ann Oncol. 2019. PMID: 31143921 Review.
-
Toxicity management with combination chemotherapy and programmed death 1/programmed death ligand 1 inhibitor therapy in advanced lung cancer.Cancer Treat Rev. 2020 Apr;85:101979. doi: 10.1016/j.ctrv.2020.101979. Epub 2020 Feb 4. Cancer Treat Rev. 2020. PMID: 32078962 Review.
Cited by
-
Perioperative chemoimmunotherapy induces strong immune responses and long-term survival in patients with HLA class I-deficient non-small cell lung cancer.J Immunother Cancer. 2024 Oct 20;12(10):e009762. doi: 10.1136/jitc-2024-009762. J Immunother Cancer. 2024. PMID: 39428126 Free PMC article.
-
Cold and hot tumors: from molecular mechanisms to targeted therapy.Signal Transduct Target Ther. 2024 Oct 18;9(1):274. doi: 10.1038/s41392-024-01979-x. Signal Transduct Target Ther. 2024. PMID: 39420203 Free PMC article. Review.
-
Single-arm trial of neoadjuvant ipilimumab plus nivolumab with chemoradiotherapy in patients with resectable and borderline resectable lung cancer: the INCREASE study.J Immunother Cancer. 2024 Sep 30;12(9):e009799. doi: 10.1136/jitc-2024-009799. J Immunother Cancer. 2024. PMID: 39349061 Free PMC article. Clinical Trial.
-
Spatial multiplexed immunofluorescence analysis reveals coordinated cellular networks associated with overall survival in metastatic osteosarcoma.Bone Res. 2024 Sep 27;12(1):55. doi: 10.1038/s41413-024-00359-z. Bone Res. 2024. PMID: 39333065 Free PMC article.
-
Advancements in TGF-β Targeting Therapies for Head and Neck Squamous Cell Carcinoma.Cancers (Basel). 2024 Aug 31;16(17):3047. doi: 10.3390/cancers16173047. Cancers (Basel). 2024. PMID: 39272905 Free PMC article. Review.
References
-
- Yamane Y, Ishii G, Goto K. et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. J Thorac Oncol 2010; 5(1): 49–55. - PubMed
-
- Liu-Jarin X, Stoopler MB, Raftopoulos H. et al. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol 2003; 16(11): 1102. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

