Deletion of Specific Sphingolipids in Distinct Neurons Improves Spatial Memory in a Mouse Model of Alzheimer's Disease
- PMID: 29973867
- PMCID: PMC6019486
- DOI: 10.3389/fnmol.2018.00206
Deletion of Specific Sphingolipids in Distinct Neurons Improves Spatial Memory in a Mouse Model of Alzheimer's Disease
Abstract
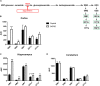
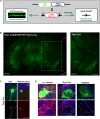
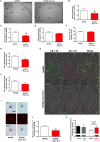
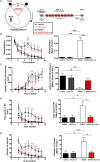
Alzheimer's disease (AD) is characterized by progressive neurodegeneration and a concomitant loss of synapses and cognitive abilities. Recently, we have proposed that an alteration of neuronal membrane lipid microdomains increases neuronal resistance toward amyloid-β stress in cultured neurons and protects from neurodegeneration in a mouse model of AD. Lipid microdomains are highly enriched in a specific subclass of glycosphingolipids, termed gangliosides. The enzyme glucosylceramide synthase (GCS) catalyzes the rate-limiting step in the biosynthesis of these gangliosides. The present work now demonstrates that genetic GCS deletion in subsets of adult forebrain neurons significantly improves the spatial memory and counteracts the loss of dendritic spines in the hippocampal dentate gyrus of 5x familial AD mice (5xFAD//Ugcgf/f//Thy1-CreERT2//EYFP mice), when compared to 5xFAD//Ugcgf/f littermates (5xFAD mice). Aberrantly activated glial cells and their expression of pro-inflammatory cytokines have emerged as the major culprits for synaptic loss in AD. Typically, astrocytic activation is accompanied by a thickening of astrocytic processes, which impairs astrocytic support for neuronal synapses. In contrast to 5xFAD mice, 5xFAD//Ugcgf/f//Thy1-CreERT2//EYFP display a less pronounced thickening of astrocytic processes and a lower expression of tumor necrosis factor-α and interleukin 1-α in the hippocampus. Thus, this work further emphasizes that GCS inhibition may constitute a potential therapeutic target against AD.
Keywords: Alzheimer’s disease; gangliosides; glial cells; spatial memory; spine density.
Figures
Similar articles
-
Genetic Deletion of Tumor Necrosis Factor-α Attenuates Amyloid-β Production and Decreases Amyloid Plaque Formation and Glial Response in the 5XFAD Model of Alzheimer's Disease.J Alzheimers Dis. 2017;60(1):165-181. doi: 10.3233/JAD-170065. J Alzheimers Dis. 2017. PMID: 28826177
-
Lipid microdomain modification sustains neuronal viability in models of Alzheimer's disease.Acta Neuropathol Commun. 2016 Sep 17;4(1):103. doi: 10.1186/s40478-016-0354-z. Acta Neuropathol Commun. 2016. PMID: 27639375 Free PMC article.
-
Conditional BDNF Delivery from Astrocytes Rescues Memory Deficits, Spine Density, and Synaptic Properties in the 5xFAD Mouse Model of Alzheimer Disease.J Neurosci. 2019 Mar 27;39(13):2441-2458. doi: 10.1523/JNEUROSCI.2121-18.2019. Epub 2019 Jan 30. J Neurosci. 2019. PMID: 30700530 Free PMC article.
-
Phloroglucinol ameliorates cognitive impairments by reducing the amyloid β peptide burden and pro-inflammatory cytokines in the hippocampus of 5XFAD mice.Free Radic Biol Med. 2018 Oct;126:221-234. doi: 10.1016/j.freeradbiomed.2018.08.016. Epub 2018 Aug 15. Free Radic Biol Med. 2018. PMID: 30118828
-
Drebrin in Alzheimer's Disease.Adv Exp Med Biol. 2017;1006:203-223. doi: 10.1007/978-4-431-56550-5_12. Adv Exp Med Biol. 2017. PMID: 28865022 Review.
Cited by
-
Role of cholesterol and sphingolipids in brain development and neurological diseases.Lipids Health Dis. 2019 Jan 25;18(1):26. doi: 10.1186/s12944-019-0965-z. Lipids Health Dis. 2019. PMID: 30683111 Free PMC article. Review.
-
The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation.Membranes (Basel). 2020 Sep 9;10(9):226. doi: 10.3390/membranes10090226. Membranes (Basel). 2020. PMID: 32916822 Free PMC article. Review.
-
Gangliosides as Therapeutic Targets for Neurodegenerative Diseases.J Lipids. 2024 Apr 8;2024:4530255. doi: 10.1155/2024/4530255. eCollection 2024. J Lipids. 2024. PMID: 38623278 Free PMC article. Review.
-
Glucosylceramide synthase inhibition reduces ganglioside GM3 accumulation, alleviates amyloid neuropathology, and stabilizes remote contextual memory in a mouse model of Alzheimer's disease.Alzheimers Res Ther. 2022 Feb 1;14(1):19. doi: 10.1186/s13195-022-00966-0. Alzheimers Res Ther. 2022. PMID: 35105352 Free PMC article.
-
Senescence-related impairment of autophagy induces toxic intraneuronal amyloid-β accumulation in a mouse model of amyloid pathology.Acta Neuropathol Commun. 2023 May 17;11(1):82. doi: 10.1186/s40478-023-01578-x. Acta Neuropathol Commun. 2023. PMID: 37198698 Free PMC article.
References
-
- Ahlijanian M. K., Barrezueta N. X., Williams R. D., Jakowski A., Kowsz K. P., McCarthy S., et al. (2000). Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl. Acad. Sci. 97 2910–2915. 10.1073/pnas.040577797 - DOI - PMC - PubMed
-
- Ariga T., Itokazu Y., McDonald M. P., Hirabayashi Y., Ando S., Yu R. K. (2013). Brain gangliosides of a transgenic mouse model of Alzheimer’s disease with deficiency in GD3-synthase: expression of elevated levels of a cholinergic-specific ganglioside, GT1aα. ASN Neuro 5 141–148. 10.1042/AN20130006 - DOI - PMC - PubMed
-
- Barrow C. J., Small D. H. (eds) (2007). Abeta Peptide and Alzheimer’s Disease. London: Springer London; 10.1007/978-1-84628-440-3 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

