Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers
- PMID: 29970158
- PMCID: PMC6029413
- DOI: 10.1186/s40425-018-0376-0
Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers
Abstract
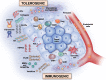
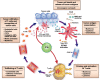
Recent advances in our understanding of the dynamics of cellular cross-talk have highlighted the significance of host-versus-tumor effect that can be harnessed with immune therapies. Tumors exploit immune checkpoints to evade adaptive immune responses. Cancer immunotherapy has witnessed a revolution in the past decade with the development of immune checkpoint inhibitors (ICIs), monoclonal antibodies against cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or their ligands, such as PD1 ligand 1 (PD-L1). ICIs have been reported to have activity against a broad range of tumor types, in both solid organ and hematologic malignancy contexts. However, less than one-third of the patients achieve a durable and meaningful treatment response. Expression of immune checkpoint ligands (e.g., PD-L1), mutational burden and tumor-infiltrating lymphocytes are currently used as biomarkers for predicting response to ICIs. However, they do not reliably predict which patients will benefit from these therapies. There is dire need to discover novel biomarkers to predict treatment efficacy and to identify areas for development of combination strategies to improve response rates. Emerging evidence suggests key roles of tumor extracellular matrix (ECM) components and their proteolytic remodeling products in regulating each step of the cancer-immunity cycle. Here we review tumor matrix dynamics and matrix remodeling in context of anti-tumor immune responses and immunotherapy and propose the exploration of matrix-based biomarkers to identify candidates for immune therapy.
Keywords: Adaptive immune response; Immune biomarkers; Immune checkpoint inhibitors; Immunotherapy; Matrix remodeling; Tumor microenvironment.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Immune checkpoint inhibitors: The linchpins of modern immunotherapy.Immunol Rev. 2019 Jul;290(1):6-23. doi: 10.1111/imr.12766. Immunol Rev. 2019. PMID: 31355494 Review.
-
Overcoming Resistance to Combination Radiation-Immunotherapy: A Focus on Contributing Pathways Within the Tumor Microenvironment.Front Immunol. 2019 Jan 31;9:3154. doi: 10.3389/fimmu.2018.03154. eCollection 2018. Front Immunol. 2019. PMID: 30766539 Free PMC article. Review.
-
Immunomodulatory effects of current cancer treatment and the consequences for follow-up immunotherapeutics.Future Oncol. 2017 Aug;13(18):1649-1663. doi: 10.2217/fon-2017-0117. Epub 2017 Aug 4. Future Oncol. 2017. PMID: 28776423 Review.
-
The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition.Int J Mol Sci. 2019 Aug 13;20(16):3934. doi: 10.3390/ijms20163934. Int J Mol Sci. 2019. PMID: 31412566 Free PMC article. Review.
-
Checkpoint blockade-based immunotherapy in the context of tumor microenvironment: Opportunities and challenges.Cancer Med. 2018 Sep;7(9):4517-4529. doi: 10.1002/cam4.1722. Epub 2018 Aug 7. Cancer Med. 2018. PMID: 30088347 Free PMC article. Review.
Cited by
-
Systematic Analysis of Aberrant Biochemical Networks and Potential Drug Vulnerabilities Induced by Tumor Suppressor Loss in Malignant Pleural Mesothelioma.Cancers (Basel). 2020 Aug 17;12(8):2310. doi: 10.3390/cancers12082310. Cancers (Basel). 2020. PMID: 32824422 Free PMC article.
-
miR-448 targets IDO1 and regulates CD8+ T cell response in human colon cancer.J Immunother Cancer. 2019 Aug 7;7(1):210. doi: 10.1186/s40425-019-0691-0. J Immunother Cancer. 2019. PMID: 31391111 Free PMC article.
-
The Cancer-Immunity Cycle in Multiple Myeloma.Immunotargets Ther. 2021 Jul 16;10:247-260. doi: 10.2147/ITT.S305432. eCollection 2021. Immunotargets Ther. 2021. PMID: 34295843 Free PMC article. Review.
-
Super-enhancer receives signals from the extracellular matrix to induce PD-L1-mediated immune evasion via integrin/BRAF/TAK1/ERK/ETV4 signaling.Cancer Biol Med. 2021 Oct 9;19(5):669-84. doi: 10.20892/j.issn.2095-3941.2021.0137. Online ahead of print. Cancer Biol Med. 2021. PMID: 34623791 Free PMC article.
-
Identification of a 14-Gene Prognostic Signature for Diffuse Large B Cell Lymphoma (DLBCL).Front Genet. 2021 Feb 10;12:625414. doi: 10.3389/fgene.2021.625414. eCollection 2021. Front Genet. 2021. PMID: 33643388 Free PMC article.
References
-
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
