Phosphodiesterase 4 inhibition reduces lung fibrosis following targeted type II alveolar epithelial cell injury
- PMID: 29952109
- PMCID: PMC6021279
- DOI: 10.14814/phy2.13753
Phosphodiesterase 4 inhibition reduces lung fibrosis following targeted type II alveolar epithelial cell injury
Abstract
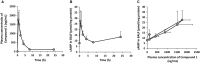
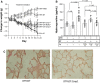
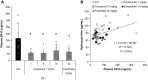
Fibrosis of the lung constitutes a major clinical challenge and novel therapies are required to alleviate the associated morbidity and mortality. Investigating the antifibrotic efficacy of drugs that are already in clinical practice offers an efficient strategy to identify new therapies. The phosphodiesterase 4 (PDE4) inhibitors, approved for the treatment of chronic obstructive pulmonary disease, harbor therapeutic potential for pulmonary fibrosis by augmenting the activity of endogenous antifibrotic mediators that signal through cyclic AMP. In this study, we tested the efficacy of several PDE4 inhibitors including a novel compound (Compound 1) in a murine model of lung fibrosis that results from a targeted type II alveolar epithelial cell injury. We also compared the antifibrotic activity of PDE4 inhibition to the two therapies that are FDA-approved for idiopathic pulmonary fibrosis (pirfenidone and nintedanib). We found that both preventative (day 0-21) and therapeutic (day 11-21) dosing regimens of the PDE4 inhibitors significantly ameliorated the weight loss and lung collagen accumulation that are the sequelae of targeted epithelial cell damage. In a therapeutic protocol, the reduction in lung fibrosis with PDE4 inhibitor administration was equivalent to pirfenidone and nintedanib. Treatment with this class of drugs also resulted in a decrease in plasma surfactant protein D concentration, a reduction in the plasma levels of several chemokines implicated in lung fibrosis, and an in vitro inhibition of fibroblast profibrotic gene expression. These results motivate further investigation of PDE4 inhibition as a treatment for patients with fibrotic lung disease.
Keywords: cAMP; collagen; epithelium; fibroblast; pulmonary.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures



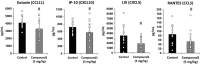



Similar articles
-
PDE4 inhibitors roflumilast and rolipram augment PGE2 inhibition of TGF-{beta}1-stimulated fibroblasts.Am J Physiol Lung Cell Mol Physiol. 2009 Jun;296(6):L959-69. doi: 10.1152/ajplung.00508.2007. Epub 2009 Mar 20. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19304913
-
The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease.Pulm Pharmacol Ther. 2010 Aug;23(4):235-56. doi: 10.1016/j.pupt.2010.03.011. Epub 2010 Apr 7. Pulm Pharmacol Ther. 2010. PMID: 20381629 Review.
-
Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages.Ann Rheum Dis. 2017 Jun;76(6):1133-1141. doi: 10.1136/annrheumdis-2016-210189. Epub 2017 Feb 16. Ann Rheum Dis. 2017. PMID: 28209630
-
Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury.Br J Pharmacol. 2009 Feb;156(3):534-44. doi: 10.1111/j.1476-5381.2008.00041.x. Br J Pharmacol. 2009. PMID: 19154443 Free PMC article.
-
Inhaled Phosphodiesterase Inhibitors for the Treatment of Chronic Obstructive Pulmonary Disease.Drugs. 2021 Nov;81(16):1821-1830. doi: 10.1007/s40265-021-01616-9. Epub 2021 Nov 3. Drugs. 2021. PMID: 34731461 Review.
Cited by
-
Pathological mechanisms and novel drug targets in fibrotic interstitial lung disease.Inflamm Regen. 2024 Jul 19;44(1):34. doi: 10.1186/s41232-024-00345-2. Inflamm Regen. 2024. PMID: 39026335 Free PMC article. Review.
-
Herbal compounds in the treatment of pulmonary silicosis.Physiol Res. 2021 Dec 31;70(S3):S275-S287. doi: 10.33549/physiolres.934817. Physiol Res. 2021. PMID: 35099247 Free PMC article. Review.
-
Design of a phase III, double-blind, randomised, placebo-controlled trial of BI 1015550 in patients with progressive pulmonary fibrosis (FIBRONEER-ILD).BMJ Open Respir Res. 2023 Sep;10(1):e001580. doi: 10.1136/bmjresp-2022-001580. BMJ Open Respir Res. 2023. PMID: 37709661 Free PMC article. Clinical Trial.
-
Classical and Emerging Therapies against Chronic Obstructive Pulmonary Disease.Chin Med J (Engl). 2018 Aug 20;131(16):1894-1897. doi: 10.4103/0366-6999.238133. Chin Med J (Engl). 2018. PMID: 30082518 Free PMC article. No abstract available.
-
Phase I studies of BI 1015550, a preferential phosphodiesterase 4B inhibitor, in healthy males and patients with idiopathic pulmonary fibrosis.ERJ Open Res. 2022 Oct 24;8(4):00240-2022. doi: 10.1183/23120541.00240-2022. eCollection 2022 Oct. ERJ Open Res. 2022. PMID: 36299369 Free PMC article.
References
-
- Borensztajn, K. , Crestani B., and Kolb M.. 2013. Idiopathic pulmonary fibrosis: from epithelial injury to biomarkers–insights from the bench side. Respiration 86:441–452. - PubMed
-
- Chaudhary, N. I. , Roth G. J., Hilberg F., Muller‐Quernheim J., Prasse A., Zissel G., et al. 2007. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 29:976–985. - PubMed
-
- Chu, Y. T. , Chang T. T., Jong Y. J., Kuo P. L., Lee H. M., Lee M. S., et al. 2010. Suppressive effects of formoterol and salmeterol on eotaxin‐1 in bronchial epithelial cells. Pediatr. Allergy Immunol. 21:345–352. - PubMed
-
- Corboz, M. R. , Zhang J., LaSala D., DiPetrillo K., Li Z., Malinin V., et al. 2018. Therapeutic administration of inhaled INS1009, a treprostinil prodrug formulation, inhibits bleomycin‐induced pulmonary fibrosis in rats. Pulm. Pharmacol. Ther. 49:95–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

