Hypoxia and IF₁ Expression Promote ROS Decrease in Cancer Cells
- PMID: 29933600
- PMCID: PMC6071258
- DOI: 10.3390/cells7070064
Hypoxia and IF₁ Expression Promote ROS Decrease in Cancer Cells
Abstract
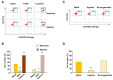
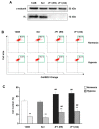
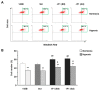
The role of reactive oxygen species (ROS) in the metabolic reprogramming of cells adapted to hypoxia and the interplay between ROS and hypoxia in malignancy is under debate. Here, we examined how ROS levels are modulated by hypoxia in human cancer compared to untransformed cells. Short time exposure (20 min) of either fibroblasts or 143B osteosarcoma cells to low oxygen tension down to 0.5% induced a significant decrease of the cellular ROS level, as detected by the CellROX fluorescent probe (−70%). Prolonging the cells’ exposure to hypoxia for 24 h, ROS decreased further, reaching nearly 20% of the normoxic value. In this regard, due to the debated role of the endogenous inhibitor protein (IF₁) of the ATP synthase complex in cancer cell bioenergetics, we investigated whether IF₁ is involved in the control of ROS generation under severe hypoxic conditions. A significant ROS content decrease was observed in hypoxia in both IF₁-expressing and IF₁- silenced cells compared to normoxia. However, IF₁-silenced cells showed higher ROS levels compared to IF1-containing cells. In addition, the MitoSOX Red-measured superoxide level of all the hypoxic cells was significantly lower compared to normoxia; however, the decrease was milder than the marked drop of ROS content. Accordingly, the difference between IF₁-expressing and IF₁-silenced cells was smaller but significant in both normoxia and hypoxia. In conclusion, the interplay between ROS and hypoxia and its modulation by IF₁ have to be taken into account to develop therapeutic strategies against cancer.
Keywords: F1F0-ATPase; IF1; ROS; cancer cells; hypoxia; osteosarcoma; superoxide radical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The inhibitor protein (IF1) of the F1F0-ATPase modulates human osteosarcoma cell bioenergetics.J Biol Chem. 2015 Mar 6;290(10):6338-48. doi: 10.1074/jbc.M114.631788. Epub 2015 Jan 20. J Biol Chem. 2015. PMID: 25605724 Free PMC article.
-
The role of the ATPase inhibitor factor 1 (IF1) in cancer cells adaptation to hypoxia and anoxia.Biochim Biophys Acta Bioenerg. 2018 Feb;1859(2):99-109. doi: 10.1016/j.bbabio.2017.10.007. Epub 2017 Oct 31. Biochim Biophys Acta Bioenerg. 2018. PMID: 29097244
-
The ATPase Inhibitory Factor 1 (IF1): A master regulator of energy metabolism and of cell survival.Biochim Biophys Acta. 2016 Aug;1857(8):1167-1182. doi: 10.1016/j.bbabio.2016.02.004. Epub 2016 Feb 12. Biochim Biophys Acta. 2016. PMID: 26876430
-
The F1Fo-ATPase inhibitor, IF1, is a critical regulator of energy metabolism in cancer cells.Biochem Soc Trans. 2021 Apr 30;49(2):815-827. doi: 10.1042/BST20200742. Biochem Soc Trans. 2021. PMID: 33929490 Review.
-
Emerging roles of hypoxia-inducible factors and reactive oxygen species in cancer and pluripotent stem cells.Kaohsiung J Med Sci. 2015 Jun;31(6):279-86. doi: 10.1016/j.kjms.2015.03.002. Epub 2015 Apr 25. Kaohsiung J Med Sci. 2015. PMID: 26043406 Review.
Cited by
-
Mechanisms, Hallmarks, and Implications of Stem Cell Quiescence.Stem Cell Reports. 2019 Jun 11;12(6):1190-1200. doi: 10.1016/j.stemcr.2019.05.012. Stem Cell Reports. 2019. PMID: 31189093 Free PMC article. Review.
-
High-grade serous tubo-ovarian cancer refined with single-cell RNA sequencing: specific cell subtypes influence survival and determine molecular subtype classification.Genome Med. 2021 Jul 9;13(1):111. doi: 10.1186/s13073-021-00922-x. Genome Med. 2021. PMID: 34238352 Free PMC article.
-
The F1Fo-ATPase inhibitor protein IF1 in pathophysiology.Front Physiol. 2022 Aug 4;13:917203. doi: 10.3389/fphys.2022.917203. eCollection 2022. Front Physiol. 2022. PMID: 35991181 Free PMC article. Review.
-
Olea europaea Leaf Phenolics Oleuropein, Hydroxytyrosol, Tyrosol, and Rutin Induce Apoptosis and Additionally Affect Temozolomide against Glioblastoma: In Particular, Oleuropein Inhibits Spheroid Growth by Attenuating Stem-like Cell Phenotype.Life (Basel). 2023 Feb 8;13(2):470. doi: 10.3390/life13020470. Life (Basel). 2023. PMID: 36836827 Free PMC article.
-
Nitric oxide-releasing micelles with intelligent targeting for enhanced anti-tumor effect of cisplatin in hypoxia.J Nanobiotechnology. 2021 Aug 16;19(1):246. doi: 10.1186/s12951-021-00989-z. J Nanobiotechnology. 2021. PMID: 34399762 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

