Sex-Specific Features of Microglia from Adult Mice
- PMID: 29924994
- PMCID: PMC6024879
- DOI: 10.1016/j.celrep.2018.05.048
Sex-Specific Features of Microglia from Adult Mice
Abstract
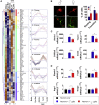
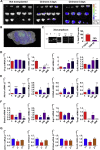
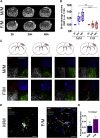
Sex has a role in the incidence and outcome of neurological illnesses, also influencing the response to treatments. Neuroinflammation is involved in the onset and progression of several neurological diseases, and the fact that estrogens have anti-inflammatory activity suggests that these hormones may be a determinant in the sex-dependent manifestation of brain pathologies. We describe significant differences in the transcriptome of adult male and female microglia, possibly originating from perinatal exposure to sex steroids. Microglia isolated from adult brains maintain the sex-specific features when put in culture or transplanted in the brain of the opposite sex. Female microglia are neuroprotective because they restrict the damage caused by acute focal cerebral ischemia. This study therefore provides insight into a distinct perspective on the mechanisms underscoring a sexual bias in the susceptibility to brain diseases.
Keywords: cell transfer; estrogens; ischemic stroke; microglia; neuroinflammation; sexual differentiation.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Microglia show sex-specific gene expression profiles.Nat Rev Neurol. 2018 Aug;14(8):452. doi: 10.1038/s41582-018-0040-9. Nat Rev Neurol. 2018. PMID: 29991822 No abstract available.
Similar articles
-
Sexual differentiation of microglia.Front Neuroendocrinol. 2019 Jan;52:156-164. doi: 10.1016/j.yfrne.2018.11.003. Epub 2018 Nov 24. Front Neuroendocrinol. 2019. PMID: 30481522 Review.
-
Microglia TREM2 is required for electroacupuncture to attenuate neuroinflammation in focal cerebral ischemia/reperfusion rats.Biochem Biophys Res Commun. 2018 Sep 18;503(4):3225-3234. doi: 10.1016/j.bbrc.2018.08.130. Epub 2018 Aug 24. Biochem Biophys Res Commun. 2018. PMID: 30149915
-
Sexually dimorphic microglia and ischemic stroke.CNS Neurosci Ther. 2019 Dec;25(12):1308-1317. doi: 10.1111/cns.13267. Epub 2019 Nov 20. CNS Neurosci Ther. 2019. PMID: 31747126 Free PMC article. Review.
-
Ginsenoside Rd Is Efficacious Against Acute Ischemic Stroke by Suppressing Microglial Proteasome-Mediated Inflammation.Mol Neurobiol. 2016 May;53(4):2529-40. doi: 10.1007/s12035-015-9261-8. Epub 2015 Jun 17. Mol Neurobiol. 2016. PMID: 26081140 Clinical Trial.
-
Stress primes microglial polarization after global ischemia: Therapeutic potential of progesterone.Brain Behav Immun. 2017 Nov;66:177-192. doi: 10.1016/j.bbi.2017.06.012. Epub 2017 Jun 23. Brain Behav Immun. 2017. PMID: 28648389
Cited by
-
Microglia Signatures: A Cause or Consequence of Microglia-Related Brain Disorders?Int J Mol Sci. 2024 Oct 11;25(20):10951. doi: 10.3390/ijms252010951. Int J Mol Sci. 2024. PMID: 39456734 Free PMC article. Review.
-
Why Do the Cosmic Rays Induce Aging?Front Physiol. 2020 Aug 12;11:955. doi: 10.3389/fphys.2020.00955. eCollection 2020. Front Physiol. 2020. PMID: 32903447 Free PMC article. Review.
-
Considerations for Studying Sex as a Biological Variable in Spinal Cord Injury.Front Neurol. 2020 Aug 5;11:802. doi: 10.3389/fneur.2020.00802. eCollection 2020. Front Neurol. 2020. PMID: 32849242 Free PMC article.
-
Cystatin F (Cst7) drives sex-dependent changes in microglia in an amyloid-driven model of Alzheimer's disease.Elife. 2023 Dec 12;12:e85279. doi: 10.7554/eLife.85279. Elife. 2023. PMID: 38085657 Free PMC article.
-
Microglia-mediated calcium-permeable AMPAR accumulation in the nucleus accumbens drives hyperlocomotion during cocaine withdrawal.Brain Behav Immun. 2024 Jan;115:535-542. doi: 10.1016/j.bbi.2023.11.007. Epub 2023 Nov 13. Brain Behav Immun. 2024. PMID: 37967660 Free PMC article.
References
-
- Arnold A.P., Gorski R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984;7:413–442. - PubMed
-
- Ciana P., Raviscioni M., Mussi P., Vegeto E., Que I., Parker M.G., Lowik C., Maggi A. In vivo imaging of transcriptionally active estrogen receptors. Nat. Med. 2003;9:82–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

