Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH
- PMID: 29920654
- PMCID: PMC6150260
- DOI: 10.1111/cei.13159
Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH
Abstract
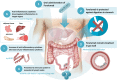
The immune system plays a role in the pathogenesis of non-alcoholic steatohepatitis (NASH) underlying hepatocyte injury and fibrosis progression at all disease stages. Oral administration of anti-CD3 monoclonal antibody (mAb) has been shown in preclinical studies to be an effective method for systemic immune modulation and alleviates immune-mediated disorders without T cell depletion. In the present review, we summarize the concept of the oral administration of humanized anti-CD3 mAb in patients with NASH and discuss the potential of this treatment to address the current requirements of treatments for NASH. Recently published preclinical and clinical data on oral administration of anti CD3 are discussed. Human trials have shown that the oral administration of anti-CD3 in healthy volunteers, patients with chronic hepatitis C virus (HCV) infection and patients with NASH and type 2 diabetes is safe and well tolerated, as well as biologically active. Oral anti-CD3 induces regulatory T cells, suppresses the chronic inflammatory state associated with NASH and exerts a beneficial effect on clinically relevant parameters. Foralumab is a fully human anti-CD3 mAb that has recently been shown to exert a potent anti-inflammatory effect in humanized mice. It is being developed for treatment of NASH and primary biliary cholangitis (PBC). Oral administration of anti CD3 may provide an effective therapy for patients with NASH.
Keywords: NAFLD; NASH; anti CD3; oral tolerance; treatment.
© 2018 British Society for Immunology.
Figures
Similar articles
-
Oral anti-CD3 immunotherapy for HCV-nonresponders is safe, promotes regulatory T cells and decreases viral load and liver enzyme levels: results of a phase-2a placebo-controlled trial.J Viral Hepat. 2015 Aug;22(8):651-7. doi: 10.1111/jvh.12369. Epub 2014 Nov 21. J Viral Hepat. 2015. PMID: 25412903 Clinical Trial.
-
Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial.J Clin Immunol. 2015 May;35(4):399-407. doi: 10.1007/s10875-015-0160-6. Epub 2015 Apr 17. J Clin Immunol. 2015. PMID: 25876706 Clinical Trial.
-
Anti-drug Antibodies Against a Novel Humanized Anti-CD20 Antibody Impair Its Therapeutic Effect on Primary Biliary Cholangitis in Human CD20- and FcγR-Expressing Mice.Front Immunol. 2018 Nov 2;9:2534. doi: 10.3389/fimmu.2018.02534. eCollection 2018. Front Immunol. 2018. PMID: 30450101 Free PMC article.
-
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside.Immunotherapy. 2016 Jul;8(8):889-906. doi: 10.2217/imt-2016-0049. Epub 2016 May 10. Immunotherapy. 2016. PMID: 27161438 Review.
-
Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression.Aliment Pharmacol Ther. 2016 Dec;44(11-12):1168-1182. doi: 10.1111/apt.13833. Epub 2016 Oct 24. Aliment Pharmacol Ther. 2016. PMID: 27778363 Free PMC article. Review.
Cited by
-
The role of bacterial translocation in sepsis: a new target for therapy.Therap Adv Gastroenterol. 2022 May 9;15:17562848221094214. doi: 10.1177/17562848221094214. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 35574428 Free PMC article. Review.
-
A digital health platform for assisting the diagnosis and monitoring of COVID-19 progression: An adjuvant approach for augmenting the antiviral response and mitigating the immune-mediated target organ damage.Biomed Pharmacother. 2021 Nov;143:112228. doi: 10.1016/j.biopha.2021.112228. Epub 2021 Sep 22. Biomed Pharmacother. 2021. PMID: 34649354 Free PMC article.
-
Workup and Management of Immune-Mediated Hepatobiliary Pancreatic Toxicities That Develop During Immune Checkpoint Inhibitor Treatment.Oncologist. 2020 Feb;25(2):105-111. doi: 10.1634/theoncologist.2018-0162. Epub 2019 Sep 9. Oncologist. 2020. PMID: 32043797 Free PMC article.
-
Inflammatory Mechanisms Underlying Nonalcoholic Steatohepatitis and the Transition to Hepatocellular Carcinoma.Cancers (Basel). 2021 Feb 10;13(4):730. doi: 10.3390/cancers13040730. Cancers (Basel). 2021. PMID: 33578800 Free PMC article. Review.
-
Delivery of Orally Administered Digestible Antibodies Using Nanoparticles.Int J Mol Sci. 2021 Mar 25;22(7):3349. doi: 10.3390/ijms22073349. Int J Mol Sci. 2021. PMID: 33805888 Free PMC article. Review.
References
-
- Wong VW, Chitturi S, Wong GL et al. Pathogenesis and novel treatment options for non‐alcoholic steatohepatitis. Lancet Gastroenterol Hepatol 2016; 1:56–67. - PubMed
-
- Musso G, Cassader M, Gambino R. Non‐alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov 2016; 15:249–74. - PubMed
-
- Perazzo H, Dufour JF. The therapeutic landscape of non‐alcoholic steatohepatitis. Liver Int 2017; 37:634–47. - PubMed
-
- Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non‐alcoholic fatty liver disease. Gut 2017; 66:180–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

