<Editors' Choice> Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing
- PMID: 29915432
- PMCID: PMC5995743
- DOI: 10.18999/nagjms.80.2.141
<Editors' Choice> Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing
Abstract
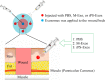
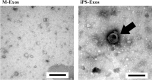
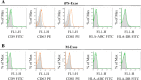
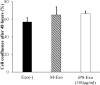
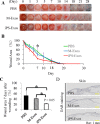
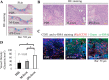
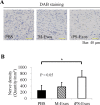
Recently, the effects of stem cell supernatants or exosomes, such as skin wounds, have attracted attention. However, the effects of the induced pluripotent stem (iPS) cell-derived exosomes (iPS-Exos) have not been investigated in detail. Here, we investigated the effects of iPS-Exos on skin wound healing using an animal model. We isolated iPS-Exos from the iPS cell culture media. Control exosomes were isolated from unused iPS cell culture media (M-Exos). We first observed the morphologic characteristics of the isolated exosomes and examined the expression of surface antigens. The effects of these exosomes on the migratory response and proliferation of fibroblasts were analyzed as well. Additionally, using a diabetic ulcer model, the effects of iPS-Exos and M-Exos on skin wound healing were investigated. Transmission electron microscope analysis demonstrated that the size of iPS-Exos (120 ± 25 nm) was significantly larger than that of M-Exos (≤ 100 nm). Flow cytometry analyses showed that iPS-Exos were positive for CD9, CD63, and CD81, whereas they were negative for HLA-ABC and -DR expression. The migratory ability of fibroblasts cocultured with iPS-Exos was shown to be higher than that of the cells cocultured with M-Exos, as demonstrated using scratch assay. Skin wound healing model results showed that the administration of iPS-Exos results in a faster wound closure compared with that observed in the M-Exo group. In conclusion, the results obtained here indicate that iPS-Exos may promote the migration of fibroblasts in vitro and in vivo, suggesting the possibility of using iPS-Exos for the treatment of diabetic ulcer.
Keywords: diabetic ulcer; exosome; induced pluripotent stem cell; wound healing.
Figures


Similar articles
-
Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis.Theranostics. 2018 Feb 7;8(6):1607-1623. doi: 10.7150/thno.22958. eCollection 2018. Theranostics. 2018. PMID: 29556344 Free PMC article.
-
[Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020 Jan 15;34(1):124-131. doi: 10.7507/1002-1892.201903058. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020. PMID: 31939247 Free PMC article. Chinese.
-
Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis.Int J Biol Sci. 2017 Feb 6;13(2):232-244. doi: 10.7150/ijbs.16951. eCollection 2017. Int J Biol Sci. 2017. PMID: 28255275 Free PMC article.
-
Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review.J Cosmet Dermatol. 2020 Mar;19(3):574-581. doi: 10.1111/jocd.13215. Epub 2019 Nov 21. J Cosmet Dermatol. 2020. PMID: 31755172 Review.
-
Exosomes from adipose-derived stem cells and application to skin wound healing.Cell Prolif. 2021 Mar;54(3):e12993. doi: 10.1111/cpr.12993. Epub 2021 Jan 17. Cell Prolif. 2021. PMID: 33458899 Free PMC article. Review.
Cited by
-
Safety and biodistribution of exosomes derived from human induced pluripotent stem cells.Front Bioeng Biotechnol. 2022 Aug 26;10:949724. doi: 10.3389/fbioe.2022.949724. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36091443 Free PMC article.
-
Photocuring 3D printing technology as an advanced tool for promoting angiogenesis in hypoxia-related diseases.J Tissue Eng. 2024 Sep 24;15:20417314241282476. doi: 10.1177/20417314241282476. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 39345255 Free PMC article. Review.
-
Exosomes: Emerging Cell-Free Based Therapeutics in Dermatologic Diseases.Front Cell Dev Biol. 2021 Oct 14;9:736022. doi: 10.3389/fcell.2021.736022. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34722517 Free PMC article. Review.
-
Human keratinocyte-derived extracellular vesicles activate the MAPKinase pathway and promote cell migration and proliferation in vitro.Inflamm Regen. 2021 Feb 2;41(1):4. doi: 10.1186/s41232-021-00154-x. Inflamm Regen. 2021. PMID: 33526070 Free PMC article.
-
Extracellular Vesicles as Potential Theranostic Platforms for Skin Diseases and Aging.Pharmaceutics. 2021 May 20;13(5):760. doi: 10.3390/pharmaceutics13050760. Pharmaceutics. 2021. PMID: 34065468 Free PMC article. Review.
References
-
- International Diabetes Federation. IDF Diabetes Atlas, (7th ed.) Brussels, Belgium: International Diabetes Federation, 2015.
-
- Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic, 2011; 12(12): 1659–1668. - PubMed
-
- Cavanagh P, Attinger C, Abbas Z, Bal A, Rojas N, Xu ZR. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev, 2012; 28 Suppl 1: 107–111. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
