Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-α-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial
- PMID: 29913017
- PMCID: PMC6233845
- DOI: 10.1001/jamaneurol.2018.1487
Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-α-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial
Abstract
Importance: Aggregated α-synuclein is believed to be central to the pathogenesis of Parkinson disease (PD). PRX002/RG7935 (PRX002) is a humanized monoclonal antibody designed to target aggregated forms of α-synuclein, thereby inhibiting neuron-to-neuron transfer of presumed pathogenic forms of α-synuclein, potentially resulting in neuronal protection and slowing disease progression.
Objective: To evaluate the safety and tolerability of multiple intravenous infusions of PRX002 in patients with idiopathic PD.
Design, setting, and participants: Multicenter, randomized, double-blind, placebo-controlled, multiple ascending-dose trial at 8 US study centers from July 2014 to September 2016. Eligible participants were aged 40 to 80 years with mild to moderate idiopathic PD (Hoehn and Yahr stages 1-3).
Interventions: Participants were enrolled into 6 ascending-dose cohorts and randomly assigned to receive PRX002 (0.3 mg/kg, 1.0 mg/kg, 3.0 mg/kg, 10 mg/kg, 30 mg/kg, or 60 mg/kg) or placebo. Participants received 3 intravenous infusions every 4 weeks of PRX002 or placebo and were monitored during a 24-week observational period.
Main outcomes and measures: Safety and tolerability assessments included physical and neurological examinations, laboratory tests, vital signs, and adverse events. Pharmacokinetic parameters included maximum PRX002 concentration, area under the curve, and half-life.
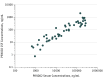
Results: Of the 80 participants, most were white (97.5%; n = 78) and male (80%; n = 64); median (SD) age was 58 (8.4) years. PRX002 was generally safe and well tolerated; no serious or severe PRX002-related treatment-emergent adverse events (TEAEs) were reported. The TEAEs experienced by at least 5% of patients receiving PRX002, irrespective of relatedness to study drug, were constipation (9.1%; n = 5), infusion reaction (7.3%; n = 4), diarrhea (5.5%; n = 3), headache (5.5%; n = 3), peripheral edema (5.5%; n = 3), post-lumbar puncture syndrome (5.5%; n = 3), and upper respiratory tract infection (5.5%; n = 3). No antidrug antibodies were detected. Serum PRX002 levels increased in an approximately dose-proportional manner; mean terminal elimination half-life was similar across all doses (10.2 days). Rapid dose- and time-dependent mean reductions from baseline vs placebo in free serum α-synuclein levels of up to 97% were seen after a single infusion at the highest dose (F78,284 = 1.66; P = .002), with similar reductions after 2 additional infusions. Mean cerebrospinal fluid PRX002 concentration increased with PRX002 dose and was approximately 0.3% relative to serum across all dose cohorts.
Conclusions and relevance: Single and multiple doses of PRX002 were generally safe and well tolerated and resulted in robust binding of peripheral α-synuclein and dose-dependent increases of PRX002 in cerebrospinal fluid, reaching cerebrospinal fluid concentrations that may be expected to engage extracellular aggregated α-synuclein in the brain. Findings support the design of an ongoing phase 2 clinical study (NCT03100149).
Trial registration: ClinicalTrials.gov Identifier: NCT02157714.
Conflict of interest statement
Figures

Comment in
-
Challenges in Passive Immunization Strategies to Treat Parkinson Disease.JAMA Neurol. 2018 Oct 1;75(10):1180-1181. doi: 10.1001/jamaneurol.2018.0346. JAMA Neurol. 2018. PMID: 29913004 No abstract available.
Similar articles
-
First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers.Mov Disord. 2017 Feb;32(2):211-218. doi: 10.1002/mds.26878. Epub 2016 Nov 25. Mov Disord. 2017. PMID: 27886407 Free PMC article. Clinical Trial.
-
Randomized Phase I Trial of the α-Synuclein Antibody Lu AF82422.Mov Disord. 2024 Jun;39(6):936-944. doi: 10.1002/mds.29784. Epub 2024 Mar 17. Mov Disord. 2024. PMID: 38494847 Clinical Trial.
-
Safety, Tolerability, and Pharmacokinetics of Single Doses of Exidavnemab (BAN0805), an Anti-α-Synuclein Antibody, in Healthy Western, Caucasian, Japanese, and Han Chinese Adults.J Clin Pharmacol. 2024 Nov;64(11):1432-1442. doi: 10.1002/jcph.6103. Epub 2024 Aug 6. J Clin Pharmacol. 2024. PMID: 39105497 Clinical Trial.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Clevidipine: a review of its use in the management of acute hypertension.Am J Cardiovasc Drugs. 2009;9(2):117-34. doi: 10.2165/00129784-200909020-00006. Am J Cardiovasc Drugs. 2009. PMID: 19331440 Review.
Cited by
-
Emerging Developments in Targeting Proteotoxicity in Neurodegenerative Diseases.CNS Drugs. 2019 Sep;33(9):883-904. doi: 10.1007/s40263-019-00657-9. CNS Drugs. 2019. PMID: 31414322 Free PMC article. Review.
-
Distribution of α-Synuclein Aggregation in the Peripheral Tissues.Neurochem Res. 2022 Dec;47(12):3627-3634. doi: 10.1007/s11064-022-03586-0. Epub 2022 Mar 28. Neurochem Res. 2022. PMID: 35348944 Review.
-
Immune system responses in Parkinson's disease: Early and dynamic.Eur J Neurosci. 2019 Feb;49(3):364-383. doi: 10.1111/ejn.14290. Epub 2018 Dec 10. Eur J Neurosci. 2019. PMID: 30474172 Free PMC article. Review.
-
The Appendix in Parkinson's Disease: From Vestigial Remnant to Vital Organ?J Parkinsons Dis. 2019;9(s2):S345-S358. doi: 10.3233/JPD-191703. J Parkinsons Dis. 2019. PMID: 31609697 Free PMC article. Review.
-
In vivo effects of the alpha-synuclein misfolding inhibitor minzasolmin supports clinical development in Parkinson's disease.NPJ Parkinsons Dis. 2023 Jul 17;9(1):114. doi: 10.1038/s41531-023-00552-7. NPJ Parkinsons Dis. 2023. PMID: 37460603 Free PMC article.
References
-
- Poewe W, Seppi K, Tanner CM, et al. . Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. - PubMed
-
- Tarakad A, Jankovic J. Diagnosis and management of Parkinson’s disease. Semin Neurol. 2017;37(2):118-126. - PubMed
-
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504-506. - PubMed
-
- Li JY, Englund E, Holton JL, et al. . Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501-503. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

