Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts
- PMID: 29890746
- PMCID: PMC6032439
- DOI: 10.3390/ijms19061715
Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts
Abstract
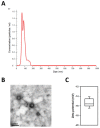
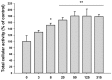
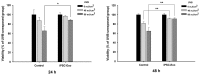
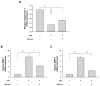
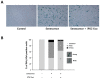
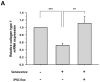
Stem cells and their paracrine factors have emerged as a resource for regenerative medicine. Many studies have shown the beneficial effects of paracrine factors secreted from adult stem cells, such as exosomes, on skin aging. However, to date, few reports have demonstrated the use of exosomes derived from human pluripotent stem cells for the treatment of skin aging. In this study, we collected exosomes from the conditioned medium of human induced pluripotent stem cells (iPSCs) and investigated the effect on aged human dermal fibroblasts (HDFs). Cell proliferation and viability were determined by an MTT assay and cell migration capacity was shown by a scratch wound assay and a transwell migration assay. To induce photoaging and natural senescence, HDFs were irradiated by UVB (315 nm) and subcultured for over 30 passages, respectively. The expression level of certain mRNAs was evaluated by quantitative real-time PCR (qPCR). Senescence-associated-β-galactosidase (SA-β-Gal) activity was assessed as a marker of natural senescence. As a result, we found that exosomes derived from human iPSCs (iPSCs-Exo) stimulated the proliferation and migration of HDFs under normal conditions. Pretreatment with iPSCs-Exo inhibited the damages of HDFs and overexpression of matrix-degrading enzymes (MMP-1/3) caused by UVB irradiation. The iPSCs-Exo also increased the expression level of collagen type I in the photo-aged HDFs. In addition, we demonstrated that iPSCs-Exo significantly reduced the expression level of SA-β-Gal and MMP-1/3 and restored the collagen type I expression in senescent HDFs. Taken together, it is anticipated that these results suggest a therapeutic potential of iPSCs-Exo for the treatment of skin aging.
Keywords: exosomes; human induced pluripotent stem cells (iPSCs); photoaging; senescence; skin regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Derivation of Cell-Engineered Nanovesicles from Human Induced Pluripotent Stem Cells and Their Protective Effect on the Senescence of Dermal Fibroblasts.Int J Mol Sci. 2020 Jan 5;21(1):343. doi: 10.3390/ijms21010343. Int J Mol Sci. 2020. PMID: 31948013 Free PMC article.
-
Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation.Int J Mol Sci. 2018 Oct 11;19(10):3119. doi: 10.3390/ijms19103119. Int J Mol Sci. 2018. PMID: 30314356 Free PMC article.
-
Antiphotoaging Effect of 3,5-Dicaffeoyl-epi-quinic Acid against UVA-Induced Skin Damage by Protecting Human Dermal Fibroblasts In Vitro.Int J Mol Sci. 2020 Oct 20;21(20):7756. doi: 10.3390/ijms21207756. Int J Mol Sci. 2020. PMID: 33092202 Free PMC article.
-
Exosomes in skin photoaging: biological functions and therapeutic opportunity.Cell Commun Signal. 2024 Jan 12;22(1):32. doi: 10.1186/s12964-023-01451-3. Cell Commun Signal. 2024. PMID: 38217034 Free PMC article. Review.
-
Human Induced Pluripotent Stem Cell-Derived Exosomes as a New Therapeutic Strategy for Various Diseases.Int J Mol Sci. 2021 Feb 10;22(4):1769. doi: 10.3390/ijms22041769. Int J Mol Sci. 2021. PMID: 33578948 Free PMC article. Review.
Cited by
-
Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells.Theranostics. 2019 Sep 21;9(23):6976-6990. doi: 10.7150/thno.35305. eCollection 2019. Theranostics. 2019. PMID: 31660081 Free PMC article.
-
Considerations and Implications in the Purification of Extracellular Vesicles - A Cautionary Tale.Front Neurosci. 2019 Oct 18;13:1067. doi: 10.3389/fnins.2019.01067. eCollection 2019. Front Neurosci. 2019. PMID: 31680809 Free PMC article.
-
Proteomic Analysis of Exosomes during Cardiogenic Differentiation of Human Pluripotent Stem Cells.Cells. 2021 Oct 1;10(10):2622. doi: 10.3390/cells10102622. Cells. 2021. PMID: 34685602 Free PMC article.
-
Neuroprotective Effects of Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cell Extracellular Vesicles in Ischemic Stroke Models.Biomedicines. 2023 Sep 17;11(9):2550. doi: 10.3390/biomedicines11092550. Biomedicines. 2023. PMID: 37760991 Free PMC article.
-
Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration.World J Stem Cells. 2022 May 26;14(5):318-329. doi: 10.4252/wjsc.v14.i5.318. World J Stem Cells. 2022. PMID: 35722196 Free PMC article. Review.
References
-
- Helfrich Y.R., Sachs D.L., Voorhees J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 2008;20:177–183. - PubMed
-
- Varani J. Fibroblast aging: intrinsic and extrinsic factors. Drug Discov. Today Ther. Strateg. 2010;7:65–70. doi: 10.1016/j.ddstr.2011.06.001. - DOI
-
- Poljšak B., Dahmane R.G., Godić A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol. Alp. Pannonica Adriat. 2012;21:33–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

