Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis
- PMID: 29867964
- PMCID: PMC5949344
- DOI: 10.3389/fimmu.2018.00987
Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis
Abstract
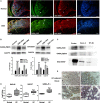
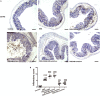
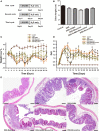
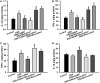
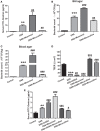
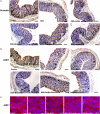
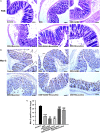
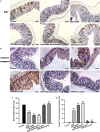
Emerging evidence indicates that gamma-aminobutyric acid (GABA) has many beneficial effects such as ameliorating immune and inflammatory response. But, here we reported that activation of GABAA receptors (GABAA Rs) aggravated dextran sulfate sodium (DSS)-induced colitis, although the expression of pro-inflammatory cytokines was inhibited. By contrast, blocking of GABAA Rs markedly alleviated DSS-induced colitis. Notably, GABAA Rs and glutamic acid decarboxylase 65/67 were significantly increased in colon mucosa of ulcerative colitis patients and the mouse model of colitis. Further studies showed that GABA treatment resulted in an increment of serum FITC-dextran following its oral administration, a decrement of transepithelial electrical resistance, and an increment of bacterial invasion, effects which were blocked by bicuculline. In addition, GABA inhibited the expression of tight junction proteins and mucin secretion in colitis colon. GABA also decreased the expression of ki-67 and increased cleaved-caspase 3 expression in intestinal epithelia. Our data indicate that the GABAA Rs activation within colon mucosa disrupts the intestinal barrier and increases the intestinal permeability which facilitates inflammatory reaction in colon. Meanwhile, the suppression effect of GABA on pro-inflammatory cytokines leads to insufficient bacteria elimination and further aggravated the bacteria invasion and inflammatory damage.
Keywords: apoptosis; epithelial proliferation; gamma-aminobutyric acid; mucosal barrier; ulcerative colitis.
Figures


Similar articles
-
Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice.BMC Gastroenterol. 2012 May 30;12:57. doi: 10.1186/1471-230X-12-57. BMC Gastroenterol. 2012. PMID: 22647055 Free PMC article.
-
Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis.J Immunol. 2015 Feb 15;194(4):1882-93. doi: 10.4049/jimmunol.1402300. Epub 2015 Jan 19. J Immunol. 2015. PMID: 25601921
-
Mucosa repair mechanisms of Tong-Xie-Yao-Fang mediated by CRH-R2 in murine, dextran sulfate sodium-induced colitis.World J Gastroenterol. 2018 Apr 28;24(16):1766-1778. doi: 10.3748/wjg.v24.i16.1766. World J Gastroenterol. 2018. PMID: 29713130 Free PMC article.
-
The GABA and GABA-Receptor System in Inflammation, Anti-Tumor Immune Responses, and COVID-19.Biomedicines. 2023 Jan 18;11(2):254. doi: 10.3390/biomedicines11020254. Biomedicines. 2023. PMID: 36830790 Free PMC article. Review.
-
What sequence of pathogenetic events leads to acute ulcerative colitis?Dis Colon Rectum. 1988 Jun;31(6):482-7. doi: 10.1007/BF02552623. Dis Colon Rectum. 1988. PMID: 3288452 Review.
Cited by
-
Inhibition of GABAAR or Application of Lactobacillus casei Zhang Alleviates Ulcerative Colitis in Mice: GABAAR as a Potential Target for Intestinal Epithelial Renewal and Repair.Int J Mol Sci. 2022 Sep 23;23(19):11210. doi: 10.3390/ijms231911210. Int J Mol Sci. 2022. PMID: 36232509 Free PMC article.
-
GABA Administration Ameliorates the Toxicity of Doxorubicin on CSF and the Brain of Albino Rats.Ann Neurosci. 2024 Jan;31(1):12-20. doi: 10.1177/09727531231161911. Epub 2023 Apr 21. Ann Neurosci. 2024. PMID: 38584977 Free PMC article.
-
Neuroimmunomodulation by gut bacteria: Focus on inflammatory bowel diseases.World J Gastrointest Pathophysiol. 2021 May 22;12(3):25-39. doi: 10.4291/wjgp.v12.i3.25. World J Gastrointest Pathophysiol. 2021. PMID: 34084590 Free PMC article. Review.
-
Intervention in gut microbiota increases intestinal γ-aminobutyric acid and alleviates anxiety behavior: a possible mechanism via the action on intestinal epithelial cells.Front Cell Infect Microbiol. 2024 Sep 5;14:1421791. doi: 10.3389/fcimb.2024.1421791. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39301289 Free PMC article.
-
PVA enema ameliorates DSS-induced acute colitis in mice.BMC Gastroenterol. 2023 Oct 30;23(1):368. doi: 10.1186/s12876-023-03005-w. BMC Gastroenterol. 2023. PMID: 37904100 Free PMC article.
References
-
- Roberts E, Frankel S. Gamma-aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem (1950) 187(1):55–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

