Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease
- PMID: 29865154
- PMCID: PMC6025525
- DOI: 10.3390/cells7060052
Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease
Abstract
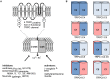
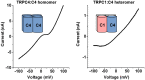
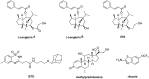
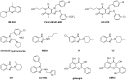
Proteins of the TRPC family can form many homo- and heterotetrameric cation channels permeable to Na⁺, K⁺ and Ca2+. In this review, we focus on channels formed by the isoforms TRPC1, TRPC4 and TRPC5. We review evidence for the formation of different TRPC1/4/5 tetramers, give an overview of recently developed small-molecule TRPC1/4/5 activators and inhibitors, highlight examples of biological roles of TRPC1/4/5 channels in different tissues and pathologies, and discuss how high-quality chemical probes of TRPC1/4/5 modulators can be used to understand the involvement of TRPC1/4/5 channels in physiological and pathophysiological processes.
Keywords: TRPC; calcium; chemical probes; ion channel; small molecules.
Conflict of interest statement
The authors declare no conflict of interest. A.M.’s PhD project is co-funded by AstraZeneca. The funders had no role in the design or preparation of this review, nor in the decision to publish it.
Figures
Similar articles
-
TRPC1 as a negative regulator for TRPC4 and TRPC5 channels.Pflugers Arch. 2019 Aug;471(8):1045-1053. doi: 10.1007/s00424-019-02289-w. Epub 2019 Jun 20. Pflugers Arch. 2019. PMID: 31222490 Review.
-
Potent, selective, and subunit-dependent activation of TRPC5 channels by a xanthine derivative.Br J Pharmacol. 2019 Oct;176(20):3924-3938. doi: 10.1111/bph.14791. Epub 2019 Sep 6. Br J Pharmacol. 2019. PMID: 31277085 Free PMC article.
-
Picomolar, selective, and subtype-specific small-molecule inhibition of TRPC1/4/5 channels.J Biol Chem. 2017 May 19;292(20):8158-8173. doi: 10.1074/jbc.M116.773556. Epub 2017 Mar 21. J Biol Chem. 2017. PMID: 28325835 Free PMC article.
-
The interaction domains of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heteromultimeric channels.Biochem Biophys Res Commun. 2016 Jun 3;474(3):476-481. doi: 10.1016/j.bbrc.2016.04.138. Epub 2016 Apr 27. Biochem Biophys Res Commun. 2016. PMID: 27131740
-
TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels.Cell Calcium. 2003 May-Jun;33(5-6):441-50. doi: 10.1016/s0143-4160(03)00055-1. Cell Calcium. 2003. PMID: 12765689 Review.
Cited by
-
Schwarzinicine A inhibits transient receptor potential canonical channels and exhibits overt vasorelaxation effects.Phytother Res. 2022 Jul;36(7):2952-2963. doi: 10.1002/ptr.7489. Epub 2022 May 10. Phytother Res. 2022. PMID: 35537691 Free PMC article.
-
Thermosensing ability of TRPC5: current knowledge and unsettled questions.J Physiol Sci. 2024 Oct 3;74(1):50. doi: 10.1186/s12576-024-00942-3. J Physiol Sci. 2024. PMID: 39363236 Free PMC article. Review.
-
Canonical transient receptor potential channels and their modulators: biology, pharmacology and therapeutic potentials.Arch Pharm Res. 2021 Apr;44(4):354-377. doi: 10.1007/s12272-021-01319-5. Epub 2021 Mar 24. Arch Pharm Res. 2021. PMID: 33763843 Free PMC article. Review.
-
Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels.Br J Pharmacol. 2019 Apr;176(7):832-846. doi: 10.1111/bph.14578. Epub 2019 Mar 6. Br J Pharmacol. 2019. PMID: 30656647 Free PMC article. Review.
-
Pharmacological Differences between Native Homomeric Transient Receptor Potential Canonical Type 4 Channels and Heteromeric Transient Receptor Potential Canonical Type 1/4 Channels in Lateral Septal Neurons.Pharmaceuticals (Basel). 2023 Sep 13;16(9):1291. doi: 10.3390/ph16091291. Pharmaceuticals (Basel). 2023. PMID: 37765099 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

