A Phase II Study of Pelareorep (REOLYSIN®) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma
- PMID: 29799479
- PMCID: PMC6025223
- DOI: 10.3390/cancers10060160
A Phase II Study of Pelareorep (REOLYSIN®) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma
Abstract
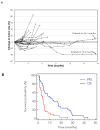
Pancreatic ductal adenocarcinoma (PDAC) has a poor prognosis, with 1 and 5-year survival rates of ~18% and 7% respectively. FOLFIRINOX or gemcitabine in combination with nab-paclitaxel are standard treatment options for metastatic disease. However, both regimens are more toxic than gemcitabine alone. Pelareorep (REOLYSIN®), a proprietary isolate of reovirus Type 3 Dearing, has shown antitumor activity in clinical and preclinical models. In addition to direct cytotoxic effects, pelareorep can trigger antitumor immune responses. Due to the high frequency of RAS mutations in PDAC, we hypothesized that pelareorep would promote selective reovirus replication in pancreatic tumors and enhance the anticancer activity of gemcitabine. Chemotherapy-naïve patients with advanced PDAC were eligible for the study. The primary objective was Clinical Benefit Rate (complete response (CR) + partial response (PR) + stable disease (SD) ≥ 12 weeks) and secondary objectives include overall survival (OS), toxicity, and pharmacodynamics (PD) analysis. The study enrolled 34 patients; results included one partial response, 23 stable disease, and 5 progressive disease. The median OS was 10.2 months, with a 1- and 2-year survival rate of 45% and 24%, respectively. The treatment was well tolerated with manageable nonhematological toxicities. PD analysis revealed reovirus replication within pancreatic tumor and associated apoptosis. Upregulation of immune checkpoint marker PD-L1 suggests future consideration of combining oncolytic virus therapy with anti-PD-L1 inhibitors. We conclude that pelareorep complements single agent gemcitabine in PDAC.
Keywords: PD-L1; REOLYSIN®; immuno-oncolytic virus; pancreatic cancer; pelareorep; reovirus.
Conflict of interest statement
Nicole Noronha, Hue Tran, Romit Chakrabarty, Giovanni Selvaggi, Andres Gutierrez, and Matt Coffey are employees of Oncolytics Biotech Inc. with stock options and/or stock. The other authors declare no conflicts of interest.
Figures


Similar articles
-
Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study.Clin Cancer Res. 2020 Jan 1;26(1):71-81. doi: 10.1158/1078-0432.CCR-19-2078. Epub 2019 Nov 6. Clin Cancer Res. 2020. PMID: 31694832 Free PMC article. Clinical Trial.
-
A phase II study of REOLYSIN® (pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant melanoma.Cancer Chemother Pharmacol. 2017 Apr;79(4):697-703. doi: 10.1007/s00280-017-3260-6. Epub 2017 Mar 13. Cancer Chemother Pharmacol. 2017. PMID: 28289863 Clinical Trial.
-
Study protocol for an open-label, single-arm, phase Ib/II study of combination of toripalimab, nab-paclitaxel, and gemcitabine as the first-line treatment for patients with unresectable pancreatic ductal adenocarcinoma.BMC Cancer. 2020 Jul 9;20(1):636. doi: 10.1186/s12885-020-07126-3. BMC Cancer. 2020. PMID: 32646394 Free PMC article.
-
The oncolytic virus, pelareorep, as a novel anticancer agent: a review.Invest New Drugs. 2015 Jun;33(3):761-74. doi: 10.1007/s10637-015-0216-8. Epub 2015 Feb 19. Invest New Drugs. 2015. PMID: 25693885 Review.
-
GOBLET: a phase I/II study of pelareorep and atezolizumab +/- chemo in advanced or metastatic gastrointestinal cancers.Future Oncol. 2022 Aug;18(26):2871-2878. doi: 10.2217/fon-2022-0453. Epub 2022 Jul 7. Future Oncol. 2022. PMID: 35796248 Review.
Cited by
-
Expanding the Spectrum of Pancreatic Cancers Responsive to Vesicular Stomatitis Virus-Based Oncolytic Virotherapy: Challenges and Solutions.Cancers (Basel). 2021 Mar 9;13(5):1171. doi: 10.3390/cancers13051171. Cancers (Basel). 2021. PMID: 33803211 Free PMC article. Review.
-
Efficacy and safety of oncolytic virus combined with chemotherapy or immune checkpoint inhibitors in solid tumor patients: A meta-analysis.Front Pharmacol. 2022 Nov 14;13:1023533. doi: 10.3389/fphar.2022.1023533. eCollection 2022. Front Pharmacol. 2022. PMID: 36452227 Free PMC article.
-
Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors.Int J Mol Sci. 2020 Oct 19;21(20):7743. doi: 10.3390/ijms21207743. Int J Mol Sci. 2020. PMID: 33086754 Free PMC article. Review.
-
Repeated dosing improves oncolytic rhabdovirus therapy in mice via interactions with intravascular monocytes.Commun Biol. 2022 Dec 19;5(1):1385. doi: 10.1038/s42003-022-04254-3. Commun Biol. 2022. PMID: 36536097 Free PMC article.
-
The Heterogeneity of the Tumor Microenvironment as Essential Determinant of Development, Progression and Therapy Response of Pancreatic Cancer.Cancers (Basel). 2021 Sep 30;13(19):4932. doi: 10.3390/cancers13194932. Cancers (Basel). 2021. PMID: 34638420 Free PMC article. Review.
References
-
- Burris H.A., 3rd, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

