Protein kinase Cα drives fibroblast activation and kidney fibrosis by stimulating autophagic flux
- PMID: 29794026
- PMCID: PMC6052200
- DOI: 10.1074/jbc.RA118.002191
Protein kinase Cα drives fibroblast activation and kidney fibrosis by stimulating autophagic flux
Abstract
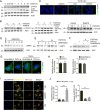
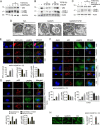
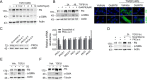
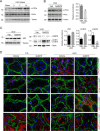
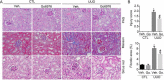
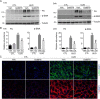
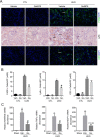
Kidney fibrosis is a histological hallmark of chronic kidney disease and arises in large part through extracellular matrix deposition by activated fibroblasts. The signaling protein complex mTOR complex 2 (mTORC2) plays a critical role in fibroblast activation and kidney fibrosis. Protein kinase Cα (PKCα) is one of the major sub-pathways of mTORC2, but its role in fibroblast activation and kidney fibrosis remains to be determined. Here, we found that transforming growth factor β1 (TGFβ1) activates PKCα signaling in cultured NRK-49F cells in a time-dependent manner. Blocking PKCα signaling with the chemical inhibitor Go6976 or by transfection with PKCα siRNA largely reduced expression of the autophagy-associated protein lysosomal-associated membrane protein 2 (LAMP2) and also inhibited autophagosome-lysosome fusion and autophagic flux in the cells. Similarly to chloroquine, Go6976 treatment and PKCα siRNA transfection also markedly inhibited TGFβ1-induced fibroblast activation. In murine fibrotic kidneys with unilateral ureteral obstruction (UUO) nephropathy, PKCα signaling is activated in the interstitial myofibroblasts. Go6976 administration largely blocked autophagic flux in fibroblasts in the fibrotic kidneys and attenuated the UUO nephropathy. Together, our findings suggest that blocking PKCα activity may retard autophagic flux and thereby prevent fibroblast activation and kidney fibrosis.
Keywords: cell signaling; fibroblast; fibrosis; kidney; signal transduction.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
Rictor/mTORC2 signaling mediates TGFβ1-induced fibroblast activation and kidney fibrosis.Kidney Int. 2015 Sep;88(3):515-27. doi: 10.1038/ki.2015.119. Epub 2015 May 13. Kidney Int. 2015. PMID: 25970154 Free PMC article.
-
Poricoic acid A activates AMPK to attenuate fibroblast activation and abnormal extracellular matrix remodelling in renal fibrosis.Phytomedicine. 2020 Jul;72:153232. doi: 10.1016/j.phymed.2020.153232. Epub 2020 May 17. Phytomedicine. 2020. PMID: 32460034
-
Transcription factor Twist1 drives fibroblast activation to promote kidney fibrosis via signaling proteins Prrx1/TNC.Kidney Int. 2024 Nov;106(5):840-855. doi: 10.1016/j.kint.2024.07.028. Epub 2024 Aug 22. Kidney Int. 2024. PMID: 39181396
-
Apamin inhibits renal fibrosis via suppressing TGF-β1 and STAT3 signaling in vivo and in vitro.J Mol Med (Berl). 2021 Sep;99(9):1265-1277. doi: 10.1007/s00109-021-02087-x. Epub 2021 May 24. J Mol Med (Berl). 2021. PMID: 34031696
-
TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis.Cell Tissue Res. 2012 Jan;347(1):117-28. doi: 10.1007/s00441-011-1181-y. Epub 2011 Jun 4. Cell Tissue Res. 2012. PMID: 21638209 Free PMC article. Review.
Cited by
-
Protein Kinase C Isozymes and Autophagy during Neurodegenerative Disease Progression.Cells. 2020 Feb 27;9(3):553. doi: 10.3390/cells9030553. Cells. 2020. PMID: 32120776 Free PMC article. Review.
-
Oxidative Stress and Inflammation in Renal and Cardiovascular Complications of Diabetes.Biology (Basel). 2020 Dec 30;10(1):18. doi: 10.3390/biology10010018. Biology (Basel). 2020. PMID: 33396868 Free PMC article. Review.
-
Fabry disease exacerbates renal interstitial fibrosis after unilateral ureteral obstruction via impaired autophagy and enhanced apoptosis.Kidney Res Clin Pract. 2021 Jun;40(2):208-219. doi: 10.23876/j.krcp.20.264. Epub 2021 May 21. Kidney Res Clin Pract. 2021. PMID: 34024086 Free PMC article.
-
TGFβ promotes fibrosis by MYST1-dependent epigenetic regulation of autophagy.Nat Commun. 2021 Jul 20;12(1):4404. doi: 10.1038/s41467-021-24601-y. Nat Commun. 2021. PMID: 34285225 Free PMC article.
-
Models of gouty nephropathy: exploring disease mechanisms and identifying potential therapeutic targets.Front Med (Lausanne). 2024 Feb 29;11:1305431. doi: 10.3389/fmed.2024.1305431. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38487029 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

