E-cadherin in contact inhibition and cancer
- PMID: 29780167
- PMCID: PMC6119098
- DOI: 10.1038/s41388-018-0304-2
E-cadherin in contact inhibition and cancer
Abstract
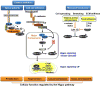
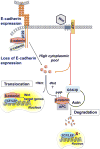
E-cadherin is a key component of the adherens junctions that are integral in cell adhesion and maintaining epithelial phenotype of cells. Homophilic E-cadherin binding between cells is important in mediating contact inhibition of proliferation when cells reach confluence. Loss of E-cadherin expression results in loss of contact inhibition and is associated with increased cell motility and advanced stages of cancer. In this review we discuss the role of E-cadherin and its downstream signaling in regulation of contact inhibition and the development and progression of cancer.
Conflict of interest statement
The authors have no conflict of interest to disclose
Figures


Similar articles
-
[Morphology, cell-cell interactions, and migratory activity of IAR-2 epithelial cells transformed with the RAS oncogene: contribution of cell adhesion protein E-cadherin].Ontogenez. 2011 Nov-Dec;42(6):453-64. Ontogenez. 2011. PMID: 22288108 Russian.
-
Dominant-negative E-cadherin alters adhesion and reverses contact inhibition of growth in breast carcinoma cells.Int J Oncol. 2002 Jul;21(1):135-44. Int J Oncol. 2002. PMID: 12063560
-
A role for atm in E-cadherin-mediated contact inhibition in epithelial cells.Breast Cancer Res Treat. 2006 Sep;99(2):143-53. doi: 10.1007/s10549-006-9195-y. Epub 2006 Mar 16. Breast Cancer Res Treat. 2006. PMID: 16541306
-
Cadherin-mediated cell-cell interactions in normal and cancer cells.Tissue Barriers. 2017 Jul 3;5(3):e1356900. doi: 10.1080/21688370.2017.1356900. Epub 2017 Jul 20. Tissue Barriers. 2017. PMID: 28783415 Free PMC article. Review.
-
Dishonorable discharge: the oncogenic roles of cleaved E-cadherin fragments.Cancer Res. 2012 Jun 15;72(12):2917-23. doi: 10.1158/0008-5472.CAN-11-3498. Epub 2012 Jun 1. Cancer Res. 2012. PMID: 22659456 Free PMC article. Review.
Cited by
-
A combined fragment-based virtual screening and STD-NMR approach for the identification of E-cadherin ligands.Front Chem. 2022 Aug 19;10:946087. doi: 10.3389/fchem.2022.946087. eCollection 2022. Front Chem. 2022. PMID: 36059878 Free PMC article.
-
Cardamonin inhibits the progression of oesophageal cancer by inhibiting the PI3K/AKT signalling pathway.J Cancer. 2021 Apr 24;12(12):3597-3610. doi: 10.7150/jca.55519. eCollection 2021. J Cancer. 2021. PMID: 33995637 Free PMC article.
-
GAS5 attenuates the malignant progression of glioma stem-like cells by promoting E-cadherin.Cancer Gene Ther. 2023 Mar;30(3):450-461. doi: 10.1038/s41417-022-00566-y. Epub 2022 Dec 2. Cancer Gene Ther. 2023. PMID: 36460802
-
Effects of cadherin mediated contact normalization on oncogenic Src kinase mediated gene expression and protein phosphorylation.Sci Rep. 2024 Oct 13;14(1):23942. doi: 10.1038/s41598-024-75449-3. Sci Rep. 2024. PMID: 39397108 Free PMC article.
-
The significance of an immunohistochemical marker-based panel in assisting the diagnosis of parathyroid carcinoma.Endocrine. 2024 Jun;84(3):1146-1153. doi: 10.1007/s12020-024-03687-6. Epub 2024 Feb 10. Endocrine. 2024. PMID: 38340242 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

