Acute intermittent hypoxia and rehabilitative training following cervical spinal injury alters neuronal hypoxia- and plasticity-associated protein expression
- PMID: 29775479
- PMCID: PMC5959066
- DOI: 10.1371/journal.pone.0197486
Acute intermittent hypoxia and rehabilitative training following cervical spinal injury alters neuronal hypoxia- and plasticity-associated protein expression
Abstract
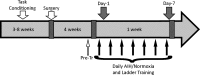
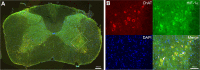
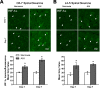
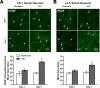
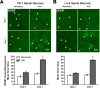
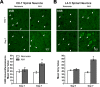
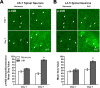
One of the most promising approaches to improve recovery after spinal cord injury (SCI) is the augmentation of spontaneously occurring plasticity in uninjured neural pathways. Acute intermittent hypoxia (AIH, brief exposures to reduced O2 levels alternating with normal O2 levels) initiates plasticity in respiratory systems and has been shown to improve recovery in respiratory and non-respiratory spinal systems after SCI in experimental animals and humans. Although the mechanism by which AIH elicits its effects after SCI are not well understood, AIH is known to alter protein expression in spinal neurons in uninjured animals. Here, we examine hypoxia- and plasticity-related protein expression using immunofluorescence in spinal neurons in SCI rats that were treated with AIH combined with motor training, a protocol which has been demonstrated to improve recovery of forelimb function in this lesion model. Specifically, we assessed protein expression in spinal neurons from animals with incomplete cervical SCI which were exposed to AIH treatment + motor training either for 1 or 7 days. AIH treatment consisted of 10 episodes of AIH: (5 min 11% O2: 5 min 21% O2) for 7 days beginning at 4 weeks post-SCI. Both 1 or 7 days of AIH treatment + motor training resulted in significantly increased expression of the transcription factor hypoxia-inducible factor-1α (HIF-1α) relative to normoxia-treated controls, in neurons both proximal (cervical) and remote (lumbar) to the SCI. All other markers examined were significantly elevated in the 7 day AIH + motor training group only, at both cervical and lumbar levels. These markers included vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and phosphorylated and nonphosphorylated forms of the BDNF receptor tropomyosin-related kinase B (TrkB). In summary, AIH induces plasticity at the cellular level after SCI by altering the expression of major plasticity- and hypoxia-related proteins at spinal regions proximal and remote to the SCI. These changes occur under the same AIH protocol which resulted in recovery of limb function in this animal model. Thus AIH, which induces plasticity in spinal circuitry, could also be an effective therapy to restore motor function after nervous system injury.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Delayed Intervention with Intermittent Hypoxia and Task Training Improves Forelimb Function in a Rat Model of Cervical Spinal Injury.J Neurotrauma. 2015 Sep 15;32(18):1403-12. doi: 10.1089/neu.2014.3789. Epub 2015 May 7. J Neurotrauma. 2015. PMID: 25664481
-
Prolonged acute intermittent hypoxia improves forelimb reach-to-grasp function in a rat model of chronic cervical spinal cord injury.Exp Neurol. 2021 Jun;340:113672. doi: 10.1016/j.expneurol.2021.113672. Epub 2021 Feb 27. Exp Neurol. 2021. PMID: 33652030 Review.
-
Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non-respiratory motor neurons.Neuroscience. 2016 May 13;322:479-88. doi: 10.1016/j.neuroscience.2016.02.060. Epub 2016 Mar 2. Neuroscience. 2016. PMID: 26944605 Free PMC article.
-
Daily acute intermittent hypoxia enhances serotonergic innervation of hypoglossal motor nuclei in rats with and without cervical spinal injury.Exp Neurol. 2022 Jan;347:113903. doi: 10.1016/j.expneurol.2021.113903. Epub 2021 Oct 24. Exp Neurol. 2022. PMID: 34699788 Free PMC article.
-
Spinal plasticity following intermittent hypoxia: implications for spinal injury.Ann N Y Acad Sci. 2010 Jun;1198:252-9. doi: 10.1111/j.1749-6632.2010.05499.x. Ann N Y Acad Sci. 2010. PMID: 20536940 Free PMC article. Review.
Cited by
-
Intermittent Hypoxia Training Prevents Deficient Learning-Memory Behavior in Mice Modeling Alzheimer's Disease: A Pilot Study.Front Aging Neurosci. 2021 Jul 1;13:674688. doi: 10.3389/fnagi.2021.674688. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34276338 Free PMC article.
-
Intermittent hypoxia changes the interaction of the kinin-VEGF system and impairs myocardial angiogenesis in the hypertrophic heart.Physiol Rep. 2021 May;9(9):e14863. doi: 10.14814/phy2.14863. Physiol Rep. 2021. PMID: 33991464 Free PMC article.
-
Hypoxic-inflammatory responses under acute hypoxia: In Vitro experiments and prospective observational expedition trial.Int J Mol Sci. 2020 Feb 4;21(3):1034. doi: 10.3390/ijms21031034. Int J Mol Sci. 2020. PMID: 32033172 Free PMC article.
-
Therapeutic acute intermittent hypoxia: A translational roadmap for spinal cord injury and neuromuscular disease.Exp Neurol. 2022 Jan;347:113891. doi: 10.1016/j.expneurol.2021.113891. Epub 2021 Oct 9. Exp Neurol. 2022. PMID: 34637802 Free PMC article. Review.
-
Advancing stroke recovery: unlocking the potential of cellular dynamics in stroke recovery.Cell Death Discov. 2024 Jul 11;10(1):321. doi: 10.1038/s41420-024-02049-5. Cell Death Discov. 2024. PMID: 38992073 Free PMC article. Review.
References
-
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology. 2014;29(1):39–48. doi: 10.1152/physiol.00012.2013 . - DOI - PMC - PubMed
-
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307(10):R1181–97. doi: 10.1152/ajpregu.00208.2014 ; PubMed Central PMCID: PMC4315448. - DOI - PMC - PubMed
-
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Annals of the New York Academy of Sciences. 2013;1279:143–53. doi: 10.1111/nyas.12085 ; PubMed Central PMCID: PMC3880582. - DOI - PMC - PubMed
-
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(11):2925–32. doi: 10.1523/JNEUROSCI.0148-05.2005 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

