Celastrol Attenuates the Invasion and Migration and Augments the Anticancer Effects of Bortezomib in a Xenograft Mouse Model of Multiple Myeloma
- PMID: 29773987
- PMCID: PMC5943600
- DOI: 10.3389/fphar.2018.00365
Celastrol Attenuates the Invasion and Migration and Augments the Anticancer Effects of Bortezomib in a Xenograft Mouse Model of Multiple Myeloma
Abstract
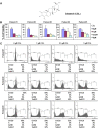
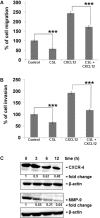
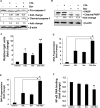
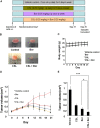
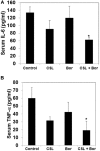
Several lines of evidence have demonstrated that deregulated activation of NF-κB plays a pivotal role in the initiation and progression of a variety of cancers including multiple myeloma (MM). Therefore, novel molecules that can effectively suppress deregulated NF-κB upregulation can potentially reduce MM growth. In this study, the effect of celastrol (CSL) on patient derived CD138+ MM cell proliferation, apoptosis, cell invasion, and migration was investigated. In addition, we studied whether CSL can potentiate the apoptotic effect of bortezomib, a proteasome inhibitor in MM cells and in a xenograft mouse model. We found that CSL significantly reduced cell proliferation and enhanced apoptosis when used in combination with bortezomib and upregulated caspase-3 in these cells. CSL also inhibited invasion and migration of MM cells through the suppression of constitutive NF-κB activation and expression of downstream gene products such as CXCR4 and MMP-9. Moreover, CSL when administered either alone or in combination with bortezomib inhibited MM tumor growth and decreased serum IL-6 and TNF-α levels. Overall, our results suggest that CSL can abrogate MM growth both in vitro and in vivo and may serve as a useful pharmacological agent for the treatment of myeloma and other hematological malignancies.
Keywords: NF-κB; bortezomib; celastrol; multiple myeloma; xenograft models.
Figures
Similar articles
-
The IAP antagonist birinapant potentiates bortezomib anti-myeloma activity in vitro and in vivo.J Hematol Oncol. 2019 Mar 7;12(1):25. doi: 10.1186/s13045-019-0713-x. J Hematol Oncol. 2019. PMID: 30845975 Free PMC article.
-
TM-233, a novel analog of 1'-acetoxychavicol acetate, induces cell death in myeloma cells by inhibiting both JAK/STAT and proteasome activities.Cancer Sci. 2015 Apr;106(4):438-46. doi: 10.1111/cas.12616. Epub 2015 Mar 10. Cancer Sci. 2015. PMID: 25613668 Free PMC article.
-
Bortezomib-enhanced radiosensitization through the suppression of radiation-induced nuclear factor-κB activity in human oral cancer cells.Int J Oncol. 2013 Mar;42(3):935-44. doi: 10.3892/ijo.2013.1786. Epub 2013 Jan 22. Int J Oncol. 2013. PMID: 23340716
-
The proteasome: a novel target for anticancer therapy.Clin Transl Oncol. 2006 May;8(5):313-7. doi: 10.1007/s12094-006-0176-8. Clin Transl Oncol. 2006. PMID: 16760005 Review.
-
The role of nuclear factor-kappaB in the biology and treatment of multiple myeloma.Semin Oncol. 2001 Dec;28(6):626-33. doi: 10.1016/s0093-7754(01)90036-3. Semin Oncol. 2001. PMID: 11740821 Review.
Cited by
-
Recent Trends in anti-tumor mechanisms and molecular targets of celastrol.Int J Biol Sci. 2024 Oct 7;20(14):5510-5530. doi: 10.7150/ijbs.99592. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494324 Free PMC article. Review.
-
PRMT5 inhibition attenuates cartilage degradation by reducing MAPK and NF-κB signaling.Arthritis Res Ther. 2020 Sep 4;22(1):201. doi: 10.1186/s13075-020-02304-x. Arthritis Res Ther. 2020. PMID: 32887644 Free PMC article.
-
Hypoxia signaling in hepatocellular carcinoma: Challenges and therapeutic opportunities.Cancer Metastasis Rev. 2023 Sep;42(3):741-764. doi: 10.1007/s10555-022-10071-1. Epub 2022 Dec 22. Cancer Metastasis Rev. 2023. PMID: 36547748 Review.
-
Pharmacological Utilization of Bergamottin, Derived from Grapefruits, in Cancer Prevention and Therapy.Int J Mol Sci. 2018 Dec 14;19(12):4048. doi: 10.3390/ijms19124048. Int J Mol Sci. 2018. PMID: 30558157 Free PMC article. Review.
-
Targeting autophagy in prostate cancer: preclinical and clinical evidence for therapeutic response.J Exp Clin Cancer Res. 2022 Mar 22;41(1):105. doi: 10.1186/s13046-022-02293-6. J Exp Clin Cancer Res. 2022. PMID: 35317831 Free PMC article. Review.
References
-
- Agarwal A., Ghobrial I. M. (2013). Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: a review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clin. Cancer Res. 19 985–994. 10.1158/1078-0432.CCR-12-2922 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

