Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC
- PMID: 29762717
- PMCID: PMC6120351
- DOI: 10.1093/neuonc/noy075
Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC
Abstract
Background: Vocimagene amiretrorepvec (Toca 511) is an investigational gamma-retroviral replicating vector encoding cytosine deaminase that, when used in combination with extended-release 5-fluorocytosine (Toca FC), results preclinically in local production of 5-fluorouracil, depletion of immune-suppressive myeloid cells, and subsequent induction of antitumor immunity. Recurrent high-grade glioma (rHGG) patients have a high unmet need for effective therapies that produce durable responses lasting more than 6 months. In this setting, relapse is nearly universal and most responses are transient.
Methods: In this Toca 511 ascending-dose phase I trial (NCT01470794), HGG patients who recurred after standard of care underwent surgical resection and received Toca 511 injected into the resection cavity wall, followed by orally administered cycles of Toca FC.
Results: Among 56 patients, durable complete responses were observed. A subgroup was identified based on Toca 511 dose and entry requirements for the follow-up phase III study. In this subgroup, which included both isocitrate dehydrogenase 1 (IDH1) mutant and wild-type tumors, the durable response rate is 21.7%. Median duration of follow-up for responders is 35.7+ months. As of August 25, 2017, all responders remain in response and are alive 33.9+ to 52.2+ months after Toca 511 administration, suggesting a positive association of durable response with overall survival.
Conclusions: Multiyear durable responses have been observed in rHGG patients treated with Toca 511 + Toca FC in a phase I trial, and the treatment will be further evaluated in a randomized phase III trial. Among IDH1 mutant patients treated at first recurrence, there may be an enrichment of complete responders.
Figures
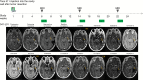
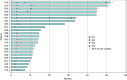
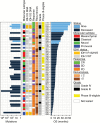
Comment in
-
Letter to the Editor.Neuro Oncol. 2019 Sep 6;21(9):1210. doi: 10.1093/neuonc/noz087. Neuro Oncol. 2019. PMID: 33064153 Free PMC article. No abstract available.
-
Reply to Dr Stoddard.Neuro Oncol. 2019 Sep 6;21(9):1211. doi: 10.1093/neuonc/noz111. Neuro Oncol. 2019. PMID: 33064154 Free PMC article. No abstract available.
Similar articles
-
Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model.Hum Gene Ther. 2015 Feb;26(2):82-93. doi: 10.1089/hum.2014.100. Epub 2015 Jan 19. Hum Gene Ther. 2015. PMID: 25419577 Free PMC article.
-
Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma.Sci Transl Med. 2016 Jun 1;8(341):341ra75. doi: 10.1126/scitranslmed.aad9784. Sci Transl Med. 2016. PMID: 27252174 Free PMC article. Clinical Trial.
-
Effect of Vocimagene Amiretrorepvec in Combination With Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients With Recurrent High-Grade Glioma: A Randomized Clinical Trial.JAMA Oncol. 2020 Dec 1;6(12):1939-1946. doi: 10.1001/jamaoncol.2020.3161. JAMA Oncol. 2020. PMID: 33119048 Free PMC article. Clinical Trial.
-
Early clinical trials of Toca 511 and Toca FC show a promising novel treatment for recurrent malignant glioma.Expert Opin Investig Drugs. 2019 Mar;28(3):207-216. doi: 10.1080/13543784.2019.1572112. Expert Opin Investig Drugs. 2019. PMID: 30676111 Review.
-
Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review.Cancer Gene Ther. 2012 Sep;19(9):593-600. doi: 10.1038/cgt.2012.36. Epub 2012 Jun 29. Cancer Gene Ther. 2012. PMID: 22744209 Review.
Cited by
-
Allogeneic Natural Killer and Cytomegalovirus (CMV)-pp65 Pulsed Dendritic Cells Induced Complete Response Through 15 Months in a Patient with Recurrent Glioblastoma: A Case Study.Am J Case Rep. 2021 Mar 31;22:e931030. doi: 10.12659/AJCR.931030. Am J Case Rep. 2021. Retraction in: Am J Case Rep. 2022 Jul 05;23:e937680. doi: 10.12659/AJCR.937680 PMID: 33788825 Free PMC article. Retracted.
-
Physiological Imaging Methods for Evaluating Response to Immunotherapies in Glioblastomas.Int J Mol Sci. 2021 Apr 8;22(8):3867. doi: 10.3390/ijms22083867. Int J Mol Sci. 2021. PMID: 33918043 Free PMC article. Review.
-
Immunotherapy of glioblastoma: Recent advances and future prospects.Hum Vaccin Immunother. 2022 Nov 30;18(5):2055417. doi: 10.1080/21645515.2022.2055417. Epub 2022 Mar 28. Hum Vaccin Immunother. 2022. PMID: 35344682 Free PMC article. Review.
-
A Critical Overview of Targeted Therapies for Glioblastoma.Front Oncol. 2018 Oct 15;8:419. doi: 10.3389/fonc.2018.00419. eCollection 2018. Front Oncol. 2018. PMID: 30374421 Free PMC article. Review.
-
Innovating Strategies and Tailored Approaches in Neuro-Oncology.Cancers (Basel). 2022 Feb 22;14(5):1124. doi: 10.3390/cancers14051124. Cancers (Basel). 2022. PMID: 35267432 Free PMC article. Review.
References
-
- Ferlay J, SI, Ervik M, et al. . GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013. http://globocan.iarc.fr. Accessed February 12, 2018.
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

