Zika virus crosses an in vitro human blood brain barrier model
- PMID: 29759080
- PMCID: PMC5952854
- DOI: 10.1186/s12987-018-0100-y
Zika virus crosses an in vitro human blood brain barrier model
Abstract
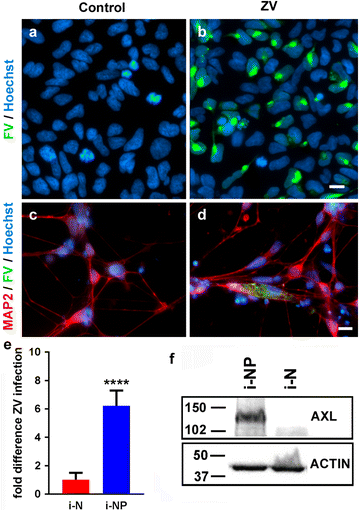
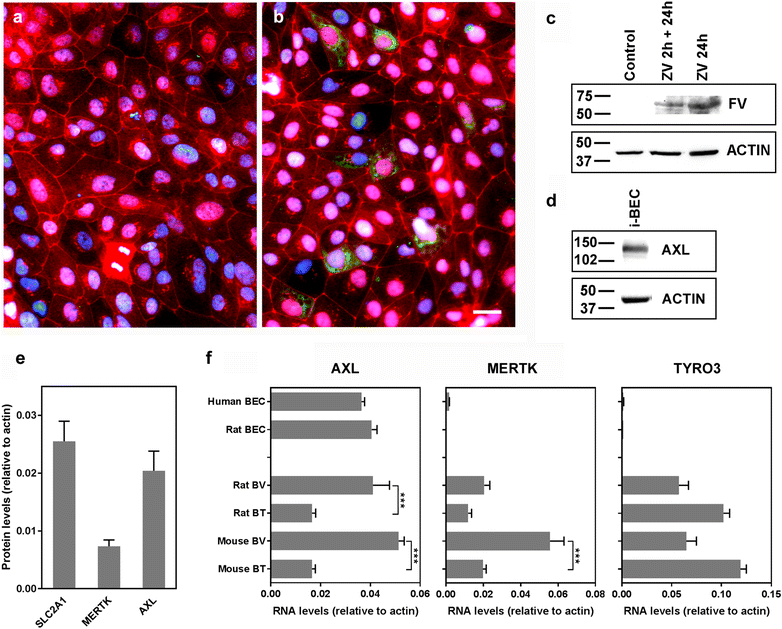
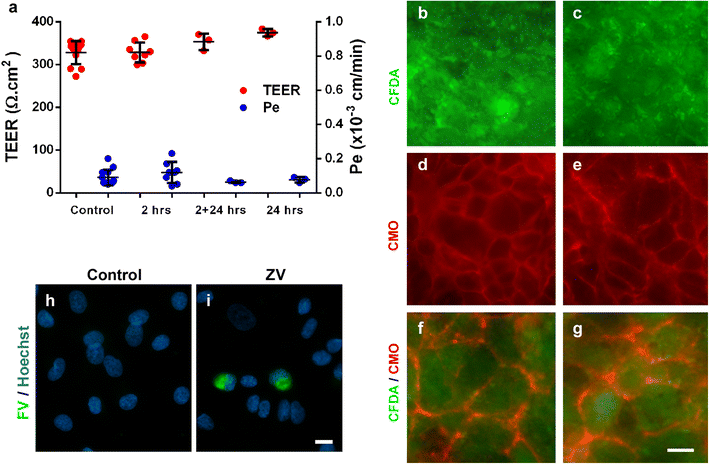
Zika virus (ZIKV) is a flavivirus that is highly neurotropic causing congenital abnormalities and neurological damage to the central nervous systems (CNS). In this study, we used a human induced pluripotent stem cell (iPSC)-derived blood brain barrier (BBB) model to demonstrate that ZIKV can infect brain endothelial cells (i-BECs) without compromising the BBB barrier integrity or permeability. Although no disruption to the BBB was observed post-infection, ZIKV particles were released on the abluminal side of the BBB model and infected underlying iPSC-derived neural progenitor cells (i-NPs). AXL, a putative ZIKV cellular entry receptor, was also highly expressed in ZIKV-susceptible i-BEC and i-NPs. This iPSC-derived BBB model can help elucidate the mechanism by which ZIKV can infect BECs, cross the BBB and gain access to the CNS.
Keywords: AXL; Blood–brain barrier model; Brain endothelial cells; Neural progenitors; Zika virus; iPSC.
Figures
Similar articles
-
Exploring Zika Virus Impact on Endothelial Permeability: Insights into Transcytosis Mechanisms and Vascular Leakage.Viruses. 2024 Apr 18;16(4):629. doi: 10.3390/v16040629. Viruses. 2024. PMID: 38675970 Free PMC article. Review.
-
Human induced pluripotent stem cell-derived glial cells and neural progenitors display divergent responses to Zika and dengue infections.Proc Natl Acad Sci U S A. 2018 Jul 3;115(27):7117-7122. doi: 10.1073/pnas.1719266115. Epub 2018 Jun 18. Proc Natl Acad Sci U S A. 2018. PMID: 29915057 Free PMC article.
-
Deciphering the Role of Extracellular Vesicles Derived from ZIKV-Infected hcMEC/D3 Cells on the Blood-Brain Barrier System.Viruses. 2021 Nov 25;13(12):2363. doi: 10.3390/v13122363. Viruses. 2021. PMID: 34960632 Free PMC article.
-
Zika Virus Infection Promotes Local Inflammation, Cell Adhesion Molecule Upregulation, and Leukocyte Recruitment at the Blood-Brain Barrier.mBio. 2020 Aug 4;11(4):e01183-20. doi: 10.1128/mBio.01183-20. mBio. 2020. PMID: 32753493 Free PMC article.
-
Zika infection and the development of neurological defects.Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12744. Epub 2017 May 3. Cell Microbiol. 2017. PMID: 28370966 Review.
Cited by
-
Zika Virus Transmission Through Blood Tissue Barriers.Front Microbiol. 2019 Jul 4;10:1465. doi: 10.3389/fmicb.2019.01465. eCollection 2019. Front Microbiol. 2019. PMID: 31333605 Free PMC article. Review.
-
Human Polymorphonuclear Cells Support Zika Virus to Cross Endothelial Monolayer and Access Bloodstream.Pathogens. 2022 Mar 5;11(3):321. doi: 10.3390/pathogens11030321. Pathogens. 2022. PMID: 35335645 Free PMC article.
-
Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System.Front Microbiol. 2019 Mar 28;10:525. doi: 10.3389/fmicb.2019.00525. eCollection 2019. Front Microbiol. 2019. PMID: 30984122 Free PMC article. Review.
-
3D engineered tissue models for studying human-specific infectious viral diseases.Bioact Mater. 2022 Sep 22;21:576-594. doi: 10.1016/j.bioactmat.2022.09.010. eCollection 2023 Mar. Bioact Mater. 2022. PMID: 36204281 Free PMC article. Review.
-
In Vitro Models of the Blood-Brain Barrier.Handb Exp Pharmacol. 2021;265:75-110. doi: 10.1007/164_2020_370. Handb Exp Pharmacol. 2021. PMID: 32562060
References
-
- Lazear HM, Diamond MS. Zika virus: new clinical syndromes and its emergence in the western hemisphere. J Virol. 2016;90:4864–75. http://www.ncbi.nlm.nih.gov/pubmed/26962217. Accessed 15 Jan 2018. - PMC - PubMed
-
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375:1–4. http://www.nejm.org/doi/10.1056/NEJMp1605367. Accessed 15 Jan 2018. - DOI - PMC - PubMed
-
- Tang BL. Zika virus as a causative agent for primary microencephaly: the evidence so far. Arch Microbiol; 2016. http://link.springer.com/10.1007/s00203-016-1268-7. Accessed 15 Jan 2018. - DOI - PubMed
-
- Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–8. http://www.nejm.org/doi/10.1056/NEJMoa1600651. Accessed 15 Jan 2018. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

