MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation
- PMID: 29757188
- PMCID: PMC5983326
- DOI: 10.1172/JCI97426
MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation
Abstract
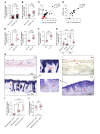
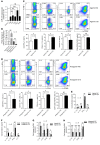
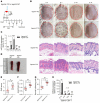
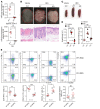
Immune imbalance of T lymphocyte subsets is a hallmark of psoriasis, but the molecular mechanisms underlying this aspect of psoriasis pathology are poorly understood. Here, we report that microRNA-210 (miR-210), a miR that is highly expressed in both psoriasis patients and mouse models, induces helper T (Th) 17 and Th1 cell differentiation but inhibits Th2 differentiation through repressing STAT6 and LYN expression, contributing to several aspects of the immune imbalance in psoriasis. Both miR-210 ablation in mice and inhibition of miR-210 by intradermal injection of antagomir-210 blocked the immune imbalance and the development of psoriasis-like inflammation in an imiquimod-induced or IL-23-induced psoriasis-like mouse model. We further showed that TGF-β and IL-23 enhance miR-210 expression by inducing HIF-1α, which recruits P300 and promotes histone H3 acetylation in the miR-210 promoter region. Our results reveal a crucial role for miR-210 in the immune imbalance of T lymphocyte subsets in psoriasis and suggest a potential therapeutic avenue.
Keywords: Autoimmune diseases; Autoimmunity; Dermatology; T cells.
Conflict of interest statement
Figures


Similar articles
-
Knockout of microRNA-155 ameliorates the Th1/Th17 immune response and tissue injury in chronic rejection.J Heart Lung Transplant. 2017 Feb;36(2):175-184. doi: 10.1016/j.healun.2016.04.018. Epub 2016 May 6. J Heart Lung Transplant. 2017. PMID: 27296836
-
MicroRNA 182 promotes T helper 1 cell by repressing hypoxia induced factor 1 alpha in experimental autoimmune encephalomyelitis.Eur J Immunol. 2019 Dec;49(12):2184-2194. doi: 10.1002/eji.201948111. Epub 2019 Sep 30. Eur J Immunol. 2019. PMID: 31411745
-
Topical administration of nanocarrier miRNA-210 antisense ameliorates imiquimod-induced psoriasis-like dermatitis in mice.J Dermatol. 2020 Feb;47(2):147-154. doi: 10.1111/1346-8138.15149. Epub 2019 Nov 27. J Dermatol. 2020. PMID: 31773789
-
Monocyte-derived interferon-alpha primed dendritic cells in the pathogenesis of psoriasis: new pieces in the puzzle.Int Immunopharmacol. 2012 Jun;13(2):215-8. doi: 10.1016/j.intimp.2012.04.003. Epub 2012 Apr 19. Int Immunopharmacol. 2012. PMID: 22522054 Review.
-
The MicroRNA-21 in Autoimmune Diseases.Int J Mol Sci. 2016 Jun 3;17(6):864. doi: 10.3390/ijms17060864. Int J Mol Sci. 2016. PMID: 27271606 Free PMC article. Review.
Cited by
-
Epigenetics in Non-tumor Immune-Mediated Skin Diseases.Mol Diagn Ther. 2021 Mar;25(2):137-161. doi: 10.1007/s40291-020-00507-1. Epub 2021 Mar 1. Mol Diagn Ther. 2021. PMID: 33646564 Review.
-
The eminent roles of ncRNAs in the pathogenesis of psoriasis.Noncoding RNA Res. 2020 Jul 3;5(3):99-108. doi: 10.1016/j.ncrna.2020.06.002. eCollection 2020 Sep. Noncoding RNA Res. 2020. PMID: 32695942 Free PMC article. Review.
-
Hypoxia-preconditioned WJ-MSC spheroid-derived exosomes delivering miR-210 for renal cell restoration in hypoxia-reoxygenation injury.Stem Cell Res Ther. 2024 Jul 30;15(1):240. doi: 10.1186/s13287-024-03845-7. Stem Cell Res Ther. 2024. PMID: 39080774 Free PMC article.
-
GDF11 Antagonizes Psoriasis-like Skin Inflammation via Suppression of NF-κB Signaling Pathway.Inflammation. 2019 Feb;42(1):319-330. doi: 10.1007/s10753-018-0895-3. Inflammation. 2019. PMID: 30259241
-
Inflammatory Cell Composition and Immune-Related microRNA Signature of Temporal Artery Biopsies From Patients With Giant Cell Arteritis.Front Immunol. 2021 Dec 23;12:791099. doi: 10.3389/fimmu.2021.791099. eCollection 2021. Front Immunol. 2021. PMID: 35003111 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

