Experimental Zika virus infection in Aedes aegypti: Susceptibility, transmission & co-infection with dengue & chikungunya viruses
- PMID: 29749366
- PMCID: PMC5967223
- DOI: 10.4103/ijmr.IJMR_1142_17
Experimental Zika virus infection in Aedes aegypti: Susceptibility, transmission & co-infection with dengue & chikungunya viruses
Abstract
Background & objectives: There are reports about the susceptibility of Aedes mosquitoes to ZIKV from various countries, however, no such information is available from Indian sub-continent, although, high level of group cross-reactivity of ZIKV with other flaviviruses has been reported. During outbreak situations, many cases of Dengue (DEN) and Chikungunya (CHIK) are reported. In such scenario, vector mosquitoes are likely to get co-infection/secondary-infection with one or other virus. The present study was carried out to determine the susceptibility of Indian strain of Aedes aegypti to Zika virus (ZIKV) strain (MR-766) and the effect of co-infection/super-infection with either dengue virus (serotype-2) (DENV) or chikungunya virus (CHIKV) on ZIKV replication.
Methods: Ae. aegypti mosquitoes used in this study were reared for many generations since 1980 at laboratory colony maintained at the ICMR-National Institute of Virology, Pune, India. Transmissibility of ZIKV from infected mosquitoes to suckling mice was also studied. Mosquitoes were experimentally infected with ZIKV and super-infected with either DENV or CHIKV via membrane-feeding route and incubated for 14 days at 28±2°C and humidity of 85±5 per cent. Replication of these viruses in mosquitoes was confirmed using real-time reverse transcription-polymerase chain reaction and immunofluorescence assay. Twenty infected mosquitoes were allowed to feed upon four suckling CD1 mice for about 30 min. Transmission of the ZIKV by infected mosquitoes to suckling mice was confirmed by the appearance of clinical signs and the presence of viral RNA in different organs.
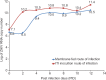
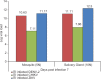
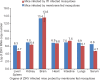
Results: Concomitant infection of mosquitoes with all the three viruses showed simultaneous propagation of all three viruses, confirmed by real time RT-PCR and IFA. Infection of mosquitoes with CHIKV followed by ZIKV showed positivity in individual head squashes (7%) for both viruses using IFA; only 8.3 per cent showed dual positivity with primary infection of ZIKV followed by DENV; 8.3 per cent dual infection positivity was observed when infected with DENV followed by ZIKV; 5 per cent showed dual infection was observed when infected with ZIKV followed by CHIKV. Ae. aegypti was found to be susceptible to ZIKV strain as ZIKV could be detected from the second post-infection day (PID) in infected mosquitoes. Transmission of ZIKV to mice by the bite of infected Ae. aegypti establishes this species as a potential vector.
Interpretation & conclusions: From super-infection experiments, it was concluded that ZIKV might have a relative advantage in replication dynamics over DENV. Vertical transmission was not observed for ZIKV in experimentally infected mosquitoes (n=920 larvae). Further studies are required to understand the possibility of silently circulating ZIKV in India, which remain non-detected because of lack of surveillance.
Keywords: Aedes aegypti; Zika; chikungunya; co-infection; dengue; transmission.
Conflict of interest statement
None
Figures
Similar articles
-
Vector competence of Aedes aegypti from Havana, Cuba, for dengue virus type 1, chikungunya, and Zika viruses.PLoS Negl Trop Dis. 2020 Dec 3;14(12):e0008941. doi: 10.1371/journal.pntd.0008941. eCollection 2020 Dec. PLoS Negl Trop Dis. 2020. PMID: 33270652 Free PMC article.
-
Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti.PLoS Negl Trop Dis. 2017 Jun 1;11(6):e0005654. doi: 10.1371/journal.pntd.0005654. eCollection 2017 Jun. PLoS Negl Trop Dis. 2017. PMID: 28570693 Free PMC article.
-
Surveillance of Aedes aegypti indoors and outdoors using Autocidal Gravid Ovitraps in South Texas during local transmission of Zika virus, 2016 to 2018.Acta Trop. 2019 Apr;192:129-137. doi: 10.1016/j.actatropica.2019.02.006. Epub 2019 Feb 11. Acta Trop. 2019. PMID: 30763563
-
Aedes vittatus (Bigot) mosquito: An emerging threat to public health.J Vector Borne Dis. 2017 Oct-Dec;54(4):295-300. doi: 10.4103/0972-9062.225833. J Vector Borne Dis. 2017. PMID: 29460858 Review.
-
Dengue and Zika viruses: lessons learned from the similarities between these Aedes mosquito-vectored arboviruses.J Microbiol. 2017 Feb;55(2):81-89. doi: 10.1007/s12275-017-6494-4. Epub 2017 Jan 26. J Microbiol. 2017. PMID: 28120186 Review.
Cited by
-
Temperature affects viral kinetics and vectorial capacity of Aedes aegypti mosquitoes co-infected with Mayaro and Dengue viruses.Parasit Vectors. 2024 Feb 19;17(1):73. doi: 10.1186/s13071-023-06109-0. Parasit Vectors. 2024. PMID: 38374048 Free PMC article.
-
Virus as Teratogenic Agents.Methods Mol Biol. 2024;2753:105-142. doi: 10.1007/978-1-0716-3625-1_4. Methods Mol Biol. 2024. PMID: 38285335
-
Aedes aegypti continuously exposed to Bacillus thuringiensis svar. israelensis does not exhibit changes in life traits but displays increased susceptibility for Zika virus.Parasit Vectors. 2021 Jul 28;14(1):379. doi: 10.1186/s13071-021-04880-6. Parasit Vectors. 2021. PMID: 34321098 Free PMC article.
-
An Overview of Indian Biomedical Research on the Chikungunya Virus with Particular Reference to Its Vaccine, an Unmet Medical Need.Vaccines (Basel). 2023 Jun 15;11(6):1102. doi: 10.3390/vaccines11061102. Vaccines (Basel). 2023. PMID: 37376491 Free PMC article. Review.
-
Arbovirus coinfection and co-transmission: A neglected public health concern?PLoS Biol. 2019 Jan 22;17(1):e3000130. doi: 10.1371/journal.pbio.3000130. eCollection 2019 Jan. PLoS Biol. 2019. PMID: 30668574 Free PMC article.
References
-
- Dick GW, Kitchen SF, Haddow AJ. Zika virus I Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

