CD147 Promotes Entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and Endothelial Cells
- PMID: 29739904
- PMCID: PMC5941078
- DOI: 10.1128/mBio.00781-18
CD147 Promotes Entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and Endothelial Cells
Abstract
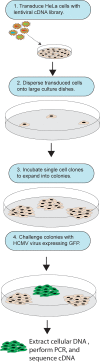
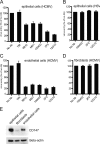
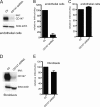
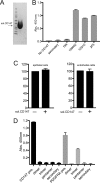
Human cytomegalovirus (HCMV) replicates in many diverse cell types in vivo, and entry into different cells involves distinct entry mechanisms and different envelope glycoproteins. HCMV glycoprotein gB is thought to act as the virus fusogen, apparently after being triggered by different gH/gL proteins that bind distinct cellular receptors or entry mediators. A trimer of gH/gL/gO is required for entry into all cell types, and entry into fibroblasts involves trimer binding to platelet-derived growth factor receptor alpha (PDGFRα). HCMV entry into biologically relevant epithelial and endothelial cells and monocyte-macrophages also requires a pentamer, gH/gL complexed with UL128, UL130, and UL131, and there is evidence that the pentamer binds unidentified receptors. We screened an epithelial cell cDNA library and identified the cell surface protein CD147, which increased entry of pentamer-expressing HCMV into HeLa cells but not entry of HCMV that lacked the pentamer. A panel of CD147-specific monoclonal antibodies inhibited HCMV entry into epithelial and endothelial cells, but not entry into fibroblasts. shRNA silencing of CD147 in endothelial cells inhibited HCMV entry but not entry into fibroblasts. CD147 colocalized with HCMV particles on cell surfaces and in endosomes. CD147 also promoted cell-cell fusion induced by expression of pentamer and gB in epithelial cells. However, soluble CD147 did not block HCMV entry and trimer and pentamer did not bind directly to CD147, supporting the hypothesis that CD147 acts indirectly through other proteins. CD147 represents the first HCMV entry mediator that specifically functions to promote entry of pentamer-expressing HCMV into epithelial and endothelial cells.IMPORTANCE Human cytomegalovirus infects nearly 80% of the world's population and causes significant morbidity and mortality. The current method of treatment involves the use of antiviral agents that are prone to resistance and can be highly toxic to patients; currently, there is no vaccine against HCMV available. HCMV infections involve virus dissemination throughout the body, infecting a wide variety of tissues; however, the mechanism of spread is not well understood, particularly with regard to which cellular proteins are utilized by HCMV to establish infection. This report describes the characterization of a newly identified cellular molecule that affects HCMV entry into epithelial and endothelial cells. These results will lead to a better understanding of HCMV pathogenesis and have implications for the development of future therapeutics.
Keywords: HCMV entry; HCMV entry mediator; HCMV pentamer; HCMV trimer.
Copyright © 2018 Vanarsdall et al.
Figures



Similar articles
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
The Human Cytomegalovirus Trimer and Pentamer Promote Sequential Steps in Entry into Epithelial and Endothelial Cells at Cell Surfaces and Endosomes.J Virol. 2018 Oct 12;92(21):e01336-18. doi: 10.1128/JVI.01336-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30111564 Free PMC article.
-
Monoclonal Antibodies to Different Components of the Human Cytomegalovirus (HCMV) Pentamer gH/gL/pUL128L and Trimer gH/gL/gO as well as Antibodies Elicited during Primary HCMV Infection Prevent Epithelial Cell Syncytium Formation.J Virol. 2016 Jun 24;90(14):6216-6223. doi: 10.1128/JVI.00121-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122579 Free PMC article.
-
Loss of the Human Cytomegalovirus US16 Protein Abrogates Virus Entry into Endothelial and Epithelial Cells by Reducing the Virion Content of the Pentamer.J Virol. 2017 May 12;91(11):e00205-17. doi: 10.1128/JVI.00205-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331097 Free PMC article.
-
Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications.Rev Med Virol. 2010 May;20(3):136-55. doi: 10.1002/rmv.645. Rev Med Virol. 2010. PMID: 20084641 Review.
Cited by
-
Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia.J Biomol Struct Dyn. 2022 Feb;40(3):1109-1119. doi: 10.1080/07391102.2020.1822208. Epub 2020 Sep 16. J Biomol Struct Dyn. 2022. PMID: 32936048 Free PMC article.
-
Identification of functionally important domains of human cytomegalovirus gO that act after trimer binding to receptors.PLoS Pathog. 2022 Apr 22;18(4):e1010452. doi: 10.1371/journal.ppat.1010452. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35452493 Free PMC article.
-
Rescue of Pentamer-Null Strains of Human Cytomegalovirus in Epithelial Cells by Use of Histone Deacetylase Inhibitors Reveals an Additional Postentry Function for the Pentamer Complex.J Virol. 2022 Apr 27;96(8):e0003122. doi: 10.1128/jvi.00031-22. Epub 2022 Mar 28. J Virol. 2022. PMID: 35343807 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
Subviral Dense Bodies of Human Cytomegalovirus Induce an Antiviral Type I Interferon Response.Cells. 2022 Dec 13;11(24):4028. doi: 10.3390/cells11244028. Cells. 2022. PMID: 36552792 Free PMC article.
References
-
- Britt W. 2006. Human cytomegalovirus infections mechanisms of disease, p 1–28. In Reddehase MJ (ed), Cytomegaloviruses: molecular biology and immunology.
-
- Pass RF. 2001. Cytomegalovirus, p 2675–2706. In Knipe DM, Howley PM (ed), Fields virology, 4th ed. Lippincott Willliams & Wilkins, ; Philadelphia, PA.
-
- Alford CA, Britt WJ. 1990. Cytomegalovirus, p 1981–2010. In Fields BN, Knipe DM, Howley PM (ed), Fields virology, 2nd ed. Raven Press, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
