Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine
- PMID: 29735652
- PMCID: PMC6003470
- DOI: 10.1073/pnas.1720758115
Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine
Abstract
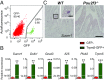
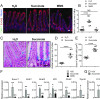
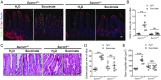
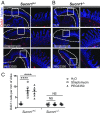
The hallmark features of type 2 mucosal immunity include intestinal tuft and goblet cell expansion initiated by tuft cell activation. How infectious agents that induce type 2 mucosal immunity are detected by tuft cells is unknown. Published microarray analysis suggested that succinate receptor 1 (Sucnr1) is specifically expressed in tuft cells. Thus, we hypothesized that the succinate-Sucnr1 axis may be utilized by tuft cells to detect certain infectious agents. Here we confirmed that Sucnr1 is specifically expressed in intestinal tuft cells but not in other types of intestinal epithelial cells, and demonstrated that dietary succinate induces tuft and goblet cell hyperplasia via Sucnr1 and the tuft cell-expressed chemosensory signaling elements gustducin and Trpm5. Conventional mice with a genetic Sucnr1 deficiency (Sucnr1-/-) showed diminished immune responses to treatment with polyethylene glycol and streptomycin, which are known to enhance microbiota-derived succinate, but responded normally to inoculation with the parasitic worm Nippostrongylus brasiliensis that also produces succinate. Thus, Sucnr1 is required for microbiota-induced but not for a generalized worm-induced type 2 immunity.
Keywords: Sucnr1; Trpm5; gustducin; tuft cells; type 2 immunity.
Conflict of interest statement
Conflict of interest statement: W.L. and P.J. have filed a patent application related to the effect of succinate on type 2 immunity.
Figures
Similar articles
-
Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit.Immunity. 2018 Jul 17;49(1):33-41.e7. doi: 10.1016/j.immuni.2018.06.016. Immunity. 2018. PMID: 30021144 Free PMC article.
-
Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites.Nature. 2016 Jan 14;529(7585):226-30. doi: 10.1038/nature16527. Nature. 2016. PMID: 26762460 Free PMC article.
-
Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity.Immunity. 2020 Mar 17;52(3):528-541.e7. doi: 10.1016/j.immuni.2020.02.005. Epub 2020 Mar 10. Immunity. 2020. PMID: 32160525 Free PMC article.
-
Interpreting heterogeneity in intestinal tuft cell structure and function.J Clin Invest. 2018 May 1;128(5):1711-1719. doi: 10.1172/JCI120330. Epub 2018 May 1. J Clin Invest. 2018. PMID: 29714721 Free PMC article. Review.
-
Regulation of immune responses by tuft cells.Nat Rev Immunol. 2019 Sep;19(9):584-593. doi: 10.1038/s41577-019-0176-x. Nat Rev Immunol. 2019. PMID: 31114038 Free PMC article. Review.
Cited by
-
Clostridium sporogenes-derived metabolites protect mice against colonic inflammation.Gut Microbes. 2024 Jan-Dec;16(1):2412669. doi: 10.1080/19490976.2024.2412669. Epub 2024 Oct 14. Gut Microbes. 2024. PMID: 39397690 Free PMC article.
-
Acetylcholine From Tuft Cells: The Updated Insights Beyond Its Immune and Chemosensory Functions.Front Cell Dev Biol. 2020 Jul 7;8:606. doi: 10.3389/fcell.2020.00606. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32733896 Free PMC article. Review.
-
Pathogenic mechanisms in the evolution of food allergy.Immunol Rev. 2024 Sep;326(1):219-226. doi: 10.1111/imr.13398. Epub 2024 Sep 17. Immunol Rev. 2024. PMID: 39285835 Free PMC article. Review.
-
Interactions between noroviruses, the host, and the microbiota.Curr Opin Virol. 2019 Aug;37:1-9. doi: 10.1016/j.coviro.2019.04.001. Epub 2019 May 13. Curr Opin Virol. 2019. PMID: 31096124 Free PMC article. Review.
-
Tuft Cells Increase Following Ovine Intestinal Parasite Infections and Define Evolutionarily Conserved and Divergent Responses.Front Immunol. 2021 Nov 22;12:781108. doi: 10.3389/fimmu.2021.781108. eCollection 2021. Front Immunol. 2021. PMID: 34880874 Free PMC article.
References
-
- Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. - PubMed
-
- Isomäki AM. A new cell type (tuft cell) in the gastrointestinal mucosa of the rat. A transmission and scanning electron microscopic study. Acta Pathol Microbiol Scand [A] 1973;240(Suppl):1–35. - PubMed
-
- Nabeyama A, Leblond CP. “Caveolated cells” characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat. 1974;140:147–165. - PubMed
-
- Höfer D, Drenckhahn D. Identification of brush cells in the alimentary and respiratory system by antibodies to villin and fimbrin. Histochemistry. 1992;98:237–242. - PubMed
-
- Hammond JB, Ladeur L. Fibrillovesicular cells in the fundic glands of the canine stomach: Evidence for a new cell type. Anat Rec. 1968;161:393–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

