The tale of two talins - two isoforms to fine-tune integrin signalling
- PMID: 29723415
- PMCID: PMC6032930
- DOI: 10.1002/1873-3468.13081
The tale of two talins - two isoforms to fine-tune integrin signalling
Abstract
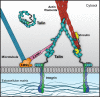
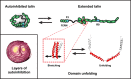
Talins are cytoplasmic adapter proteins essential for integrin-mediated cell adhesion to the extracellular matrix. Talins control the activation state of integrins, link integrins to cytoskeletal actin, recruit numerous signalling molecules that mediate integrin signalling and coordinate recruitment of microtubules to adhesion sites via interaction with KANK (kidney ankyrin repeat-containing) proteins. Vertebrates have two talin genes, TLN1 and TLN2. Although talin1 and talin2 share 76% protein sequence identity (88% similarity), they are not functionally redundant, and the differences between the two isoforms are not fully understood. In this Review, we focus on the similarities and differences between the two talins in terms of structure, biochemistry and function, which hint at subtle differences in fine-tuning adhesion signalling.
Keywords: integrin; mechanobiology; talin.
© 2018 Federation of European Biochemical Societies.
Figures

Similar articles
-
Evidence that talin alternative splice variants from Ciona intestinalis have different roles in cell adhesion.BMC Cell Biol. 2006 Dec 6;7:40. doi: 10.1186/1471-2121-7-40. BMC Cell Biol. 2006. PMID: 17150103 Free PMC article.
-
Talin2 and KANK2 functionally interact to regulate microtubule dynamics, paclitaxel sensitivity and cell migration in the MDA-MB-435S melanoma cell line.Cell Mol Biol Lett. 2023 Jul 17;28(1):56. doi: 10.1186/s11658-023-00473-6. Cell Mol Biol Lett. 2023. PMID: 37460977 Free PMC article.
-
Structural diversity in integrin/talin interactions.Structure. 2010 Dec 8;18(12):1654-66. doi: 10.1016/j.str.2010.09.018. Structure. 2010. PMID: 21134644 Free PMC article.
-
Talins and kindlins: partners in integrin-mediated adhesion.Nat Rev Mol Cell Biol. 2013 Aug;14(8):503-17. doi: 10.1038/nrm3624. Epub 2013 Jul 17. Nat Rev Mol Cell Biol. 2013. PMID: 23860236 Free PMC article. Review.
-
Talin: A Potential Drug Target for Cancer Therapy.Curr Drug Metab. 2020;21(1):25-32. doi: 10.2174/1389200221666200214114018. Curr Drug Metab. 2020. PMID: 32056520 Review.
Cited by
-
Patient genetics is linked to chronic wound microbiome composition and healing.PLoS Pathog. 2020 Jun 18;16(6):e1008511. doi: 10.1371/journal.ppat.1008511. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32555671 Free PMC article.
-
Talin mechanosensitivity is modulated by a direct interaction with cyclin-dependent kinase-1.J Biol Chem. 2021 Jul;297(1):100837. doi: 10.1016/j.jbc.2021.100837. Epub 2021 Jun 9. J Biol Chem. 2021. PMID: 34118235 Free PMC article.
-
Arterial dissections: Common features and new perspectives.Front Cardiovasc Med. 2022 Dec 6;9:1055862. doi: 10.3389/fcvm.2022.1055862. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36561772 Free PMC article. Review.
-
Talin in mechanotransduction and mechanomemory at a glance.J Cell Sci. 2021 Oct 15;134(20):jcs258749. doi: 10.1242/jcs.258749. Epub 2021 Oct 28. J Cell Sci. 2021. PMID: 34708856 Free PMC article.
-
Mutant Presenilin 1 Dysregulates Exosomal Proteome Cargo Produced by Human-Induced Pluripotent Stem Cell Neurons.ACS Omega. 2021 May 13;6(20):13033-13056. doi: 10.1021/acsomega.1c00660. eCollection 2021 May 25. ACS Omega. 2021. PMID: 34056454 Free PMC article.
References
-
- Winograd‐Katz SE, Fässler R, Geiger B and Legate KR (2014) The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol 15, 273–288. - PubMed
-
- Monkley SJ, Zhou XH, Kinston SJ, Giblett SM, Hemmings L, Priddle H, Brown JE, Pritchard CA, Critchley DR and Fässler R (2000) Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn 219, 560–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

