pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis
- PMID: 29720568
- PMCID: PMC6012518
- DOI: 10.1172/jci.insight.98380
pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis
Abstract
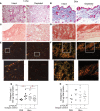
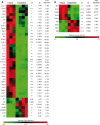
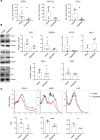
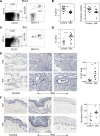
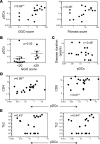
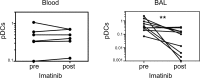
Fibrosis is the end result of most inflammatory conditions, but its pathogenesis remains unclear. We demonstrate that, in animals and humans with systemic fibrosis, plasmacytoid DCs (pDCs) are unaffected or are reduced systemically (spleen/peripheral blood), but they increase in the affected organs (lungs/skin/bronchoalveolar lavage). A pivotal role of pDCs was shown by depleting them in vivo, which ameliorated skin and/or lung fibrosis, reduced immune cell infiltration in the affected organs but not in spleen, and reduced the expression of genes and proteins implicated in chemotaxis, inflammation, and fibrosis in the affected organs of animals with bleomycin-induced fibrosis. As with animal findings, the frequency of pDCs in the lungs of patients with systemic sclerosis correlated with the severity of lung disease and with the frequency of CD4+ and IL-4+ T cells in the lung. Finally, treatment with imatinib that has been reported to reduce and/or prevent deterioration of skin and lung fibrosis profoundly reduced pDCs in lungs but not in peripheral blood of patients with systemic sclerosis. These observations suggest a role for pDCs in the pathogenesis of systemic fibrosis and identify the increased trafficking of pDCs to the affected organs as a potential therapeutic target in fibrotic diseases.
Keywords: Autoimmune diseases; Fibrosis; Inflammation; Pulmonology; Rheumatology.
Conflict of interest statement
Figures




Similar articles
-
Elevated frequencies of CD4(+) IL-21(+) T, CD4(+) IL-21R(+) T and IL-21(+) Th17 cells, and increased levels of IL-21 in bleomycin-induced mice may be associated with dermal and pulmonary inflammation and fibrosis.Int J Rheum Dis. 2016 Apr;19(4):392-404. doi: 10.1111/1756-185X.12522. Epub 2014 Dec 25. Int J Rheum Dis. 2016. PMID: 25545680
-
Low RUNX3 expression alters dendritic cell function in patients with systemic sclerosis and contributes to enhanced fibrosis.Ann Rheum Dis. 2019 Sep;78(9):1249-1259. doi: 10.1136/annrheumdis-2018-214991. Epub 2019 May 24. Ann Rheum Dis. 2019. PMID: 31126957
-
Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8.Sci Transl Med. 2018 Jan 10;10(423):eaam8458. doi: 10.1126/scitranslmed.aam8458. Sci Transl Med. 2018. PMID: 29321259 Free PMC article.
-
The Role of TLR7 and TLR9 in the Pathogenesis of Systemic Sclerosis.Int J Mol Sci. 2024 Jun 1;25(11):6133. doi: 10.3390/ijms25116133. Int J Mol Sci. 2024. PMID: 38892317 Free PMC article.
-
Tyrosine kinase inhibitors in the treatment of systemic sclerosis: from animal models to clinical trials.Curr Rheumatol Rep. 2011 Feb;13(1):21-7. doi: 10.1007/s11926-010-0142-x. Curr Rheumatol Rep. 2011. PMID: 21042889 Review.
Cited by
-
Dendritic Cell Subpopulations Are Associated with Morphological Features of Breast Ductal Carcinoma In Situ.Int J Mol Sci. 2023 Jun 8;24(12):9918. doi: 10.3390/ijms24129918. Int J Mol Sci. 2023. PMID: 37373062 Free PMC article.
-
Systemic sclerosis interstitial lung disease: unmet needs and potential solutions.Nat Rev Rheumatol. 2024 Jan;20(1):21-32. doi: 10.1038/s41584-023-01044-x. Epub 2023 Nov 3. Nat Rev Rheumatol. 2024. PMID: 37923862 Review.
-
Toll-like receptors in mediating pathogenesis in systemic sclerosis.Clin Exp Immunol. 2020 Jul;201(1):14-24. doi: 10.1111/cei.13426. Epub 2020 Feb 21. Clin Exp Immunol. 2020. PMID: 32048277 Free PMC article. Review.
-
Immune and Non-Immune Inflammatory Cells Involved in Autoimmune Fibrosis: New Discoveries.J Clin Med. 2023 May 31;12(11):3801. doi: 10.3390/jcm12113801. J Clin Med. 2023. PMID: 37297996 Free PMC article. Review.
-
Etiology, Risk Factors, and Biomarkers in Systemic Sclerosis with Interstitial Lung Disease.Am J Respir Crit Care Med. 2020 Mar 15;201(6):650-660. doi: 10.1164/rccm.201903-0563CI. Am J Respir Crit Care Med. 2020. PMID: 31841044 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

