Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy
- PMID: 29717119
- PMCID: PMC5931527
- DOI: 10.1038/s41467-018-04038-6
Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy
Abstract
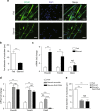
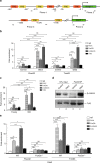
Age-related muscle atrophy (sarcopenia) is the leading cause for disability in aged population, but the underlying molecular mechanisms are poorly understood. Here we identify a novel role for the secreted glycoprotein Dickkopf 3 (Dkk3) in sarcopenia. Forced expression of Dkk3 in muscles in young mice leads to muscle atrophy. Conversely, reducing its expression in old muscles restores both muscle size and function. Dkk3 induces nuclear import of β-catenin and enhances its interaction with FoxO3, which in turn activates the transcription of E3 ubiquitin ligase Fbxo32 and Trim63, driving muscle atrophy. These findings suggest that Dkk3 may be used as diagnostic marker and as therapeutic target for age-related muscle atrophy, and reveal a distinct transcriptional control of Fbxo32 and Trim63.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Iron-induced skeletal muscle atrophy involves an Akt-forkhead box O3-E3 ubiquitin ligase-dependent pathway.J Trace Elem Med Biol. 2016 May;35:66-76. doi: 10.1016/j.jtemb.2016.01.011. Epub 2016 Jan 28. J Trace Elem Med Biol. 2016. PMID: 27049128
-
Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushing's syndrome.Am J Physiol Endocrinol Metab. 2017 Jun 1;312(6):E495-E507. doi: 10.1152/ajpendo.00389.2016. Epub 2017 Feb 28. Am J Physiol Endocrinol Metab. 2017. PMID: 28246104
-
Identification of a KLF5-dependent program and drug development for skeletal muscle atrophy.Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2102895118. doi: 10.1073/pnas.2102895118. Proc Natl Acad Sci U S A. 2021. PMID: 34426497 Free PMC article.
-
The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass.Free Radic Biol Med. 2016 Sep;98:218-230. doi: 10.1016/j.freeradbiomed.2015.12.031. Epub 2015 Dec 29. Free Radic Biol Med. 2016. PMID: 26738803 Review.
-
Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy.Am J Pathol. 2022 Dec;192(12):1648-1657. doi: 10.1016/j.ajpath.2022.09.003. Epub 2022 Sep 27. Am J Pathol. 2022. PMID: 36174679 Review.
Cited by
-
An efficient protocol for studying human pluripotent stem cell-derived myotube senescence.Biophys Rep. 2023 Oct 31;9(5):232-240. doi: 10.52601/bpr.2023.230013. Biophys Rep. 2023. PMID: 38516297 Free PMC article.
-
Future Data Points to Implement in Adult Spinal Deformity Assessment for Artificial Intelligence Modeling Prediction: The Importance of the Biological Dimension.Int J Spine Surg. 2023 Jun;17(S1):S34-S44. doi: 10.14444/8502. Epub 2023 May 10. Int J Spine Surg. 2023. PMID: 37164480 Free PMC article.
-
Vitamin D sufficiency enhances differentiation of patient-derived prostate epithelial organoids.iScience. 2021 Jan 5;24(1):101974. doi: 10.1016/j.isci.2020.101974. eCollection 2021 Jan 22. iScience. 2021. PMID: 33458620 Free PMC article.
-
The landscape of aging.Sci China Life Sci. 2022 Dec;65(12):2354-2454. doi: 10.1007/s11427-022-2161-3. Epub 2022 Sep 2. Sci China Life Sci. 2022. PMID: 36066811 Free PMC article. Review.
-
High-resolution genome-wide expression analysis of single myofibers using SMART-Seq.J Biol Chem. 2019 Dec 27;294(52):20097-20108. doi: 10.1074/jbc.RA119.011506. Epub 2019 Nov 21. J Biol Chem. 2019. PMID: 31753917 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

