Exercise induces new cardiomyocyte generation in the adult mammalian heart
- PMID: 29695718
- PMCID: PMC5916892
- DOI: 10.1038/s41467-018-04083-1
Exercise induces new cardiomyocyte generation in the adult mammalian heart
Abstract
Loss of cardiomyocytes is a major cause of heart failure, and while the adult heart has a limited capacity for cardiomyogenesis, little is known about what regulates this ability or whether it can be effectively harnessed. Here we show that 8 weeks of running exercise increase birth of new cardiomyocytes in adult mice (~4.6-fold). New cardiomyocytes are identified based on incorporation of 15N-thymidine by multi-isotope imaging mass spectrometry (MIMS) and on being mononucleate/diploid. Furthermore, we demonstrate that exercise after myocardial infarction induces a robust cardiomyogenic response in an extended border zone of the infarcted area. Inhibition of miR-222, a microRNA increased by exercise in both animal models and humans, completely blocks the cardiomyogenic exercise response. These findings demonstrate that cardiomyogenesis can be activated by exercise in the normal and injured adult mouse heart and suggest that stimulation of endogenous cardiomyocyte generation could contribute to the benefits of exercise.
Conflict of interest statement
The authors declare no competing interests.
Figures
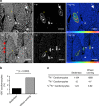

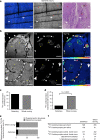
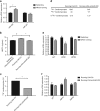
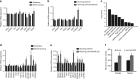
Similar articles
-
Restoration of Cardiomyogenesis in Aged Mouse Hearts by Voluntary Exercise.Circulation. 2022 Aug 2;146(5):412-426. doi: 10.1161/CIRCULATIONAHA.121.057276. Epub 2022 Jul 6. Circulation. 2022. PMID: 35862076 Free PMC article.
-
miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling.Cell Metab. 2015 Apr 7;21(4):584-95. doi: 10.1016/j.cmet.2015.02.014. Cell Metab. 2015. PMID: 25863248 Free PMC article.
-
Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure.Circ Res. 2007 Jun 22;100(12):1741-8. doi: 10.1161/CIRCRESAHA.107.153544. Epub 2007 May 10. Circ Res. 2007. PMID: 17495221
-
Formation of New Cardiomyocytes in Exercise.Adv Exp Med Biol. 2017;999:91-102. doi: 10.1007/978-981-10-4307-9_6. Adv Exp Med Biol. 2017. PMID: 29022259 Review.
-
The Molecular Mechanisms Associated with Aerobic Exercise-Induced Cardiac Regeneration.Biomolecules. 2020 Dec 27;11(1):19. doi: 10.3390/biom11010019. Biomolecules. 2020. PMID: 33375497 Free PMC article. Review.
Cited by
-
Non-coding RNAs in Cardiac Regeneration.Front Physiol. 2021 Mar 24;12:650566. doi: 10.3389/fphys.2021.650566. eCollection 2021. Front Physiol. 2021. PMID: 33841185 Free PMC article. Review.
-
The healing effects of moderate exercise on acetic acid-induced gastric ulcer in male rats.Gastroenterol Hepatol Bed Bench. 2024;17(3):313-319. doi: 10.22037/ghfbb.v17i3.2948. Gastroenterol Hepatol Bed Bench. 2024. PMID: 39308531 Free PMC article.
-
Beyond cardiomyocytes: Cellular diversity in the heart's response to exercise.J Sport Health Sci. 2023 Jul;12(4):423-437. doi: 10.1016/j.jshs.2022.12.011. Epub 2022 Dec 19. J Sport Health Sci. 2023. PMID: 36549585 Free PMC article. Review.
-
Reawakening the Intrinsic Cardiac Regenerative Potential: Molecular Strategies to Boost Dedifferentiation and Proliferation of Endogenous Cardiomyocytes.Front Cardiovasc Med. 2021 Oct 8;8:750604. doi: 10.3389/fcvm.2021.750604. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34692797 Free PMC article. Review.
-
Intrinsic and Extrinsic Contributors to the Cardiac Benefits of Exercise.JACC Basic Transl Sci. 2023 Oct 18;9(4):535-552. doi: 10.1016/j.jacbts.2023.07.011. eCollection 2024 Apr. JACC Basic Transl Sci. 2023. PMID: 38680954 Free PMC article. Review.
References
-
- Rumyantsev PP. Interrelations of the proliferation and differentiation processes during cardiac myogenesis and regeneration. Int. Rev. Cytol. 1977;51:186–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

