Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages
- PMID: 29694888
- PMCID: PMC6392449
- DOI: 10.1016/j.celrep.2018.03.109
Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages
Erratum in
-
Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages.Cell Rep. 2019 Sep 17;28(12):3285. doi: 10.1016/j.celrep.2019.08.080. Cell Rep. 2019. PMID: 31533048 Free PMC article. No abstract available.
Abstract
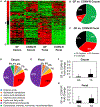
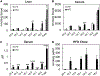
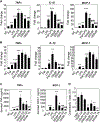
The gut microbiota plays a significant role in the progression of fatty liver disease; however, the mediators and their mechanisms remain to be elucidated. Comparing metabolite profile differences between germ-free and conventionally raised mice against differences between mice fed a low- and high-fat diet (HFD), we identified tryptamine and indole-3-acetate (I3A) as metabolites that depend on the microbiota and are depleted under a HFD. Both metabolites reduced fatty-acid- and LPS-stimulated production of pro-inflammatory cytokines in macrophages and inhibited the migration of cells toward a chemokine, with I3A exhibiting greater potency. In hepatocytes, I3A attenuated inflammatory responses under lipid loading and reduced the expression of fatty acid synthase and sterol regulatory element-binding protein-1c. These effects were abrogated in the presence of an aryl-hydrocarbon receptor (AhR) antagonist, indicating that the effects are AhR dependent. Our results suggest that gut microbiota could influence inflammatory responses in the liver through metabolites engaging host receptors.
Keywords: aryl hydrocarbon receptor; gut microbiota; indole-3-acetate; inflammation; metabolomics; nonalcoholic fatty liver disease.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures
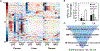



Similar articles
-
Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE-/- mice.Biomed Pharmacother. 2019 May;113:108753. doi: 10.1016/j.biopha.2019.108753. Epub 2019 Mar 9. Biomed Pharmacother. 2019. PMID: 30856537
-
Oral supplementation of gut microbial metabolite indole-3-acetate alleviates diet-induced steatosis and inflammation in mice.Elife. 2024 Feb 27;12:RP87458. doi: 10.7554/eLife.87458. Elife. 2024. PMID: 38412016 Free PMC article.
-
Microbiota-Derived Metabolites, Indole-3-aldehyde and Indole-3-acetic Acid, Differentially Modulate Innate Cytokines and Stromal Remodeling Processes Associated with Autoimmune Arthritis.Int J Mol Sci. 2021 Feb 18;22(4):2017. doi: 10.3390/ijms22042017. Int J Mol Sci. 2021. PMID: 33670600 Free PMC article.
-
Activation of aryl hydrocarbon receptor (AhR) in Alzheimer's disease: role of tryptophan metabolites generated by gut host-microbiota.J Mol Med (Berl). 2023 Mar;101(3):201-222. doi: 10.1007/s00109-023-02289-5. Epub 2023 Feb 9. J Mol Med (Berl). 2023. PMID: 36757399 Free PMC article. Review.
-
The aryl hydrocarbon receptor as a mediator of host-microbiota interplay.Gut Microbes. 2020 Nov 9;12(1):1859812. doi: 10.1080/19490976.2020.1859812. Epub 2020 Dec 17. Gut Microbes. 2020. PMID: 33382356 Free PMC article. Review.
Cited by
-
Indole-3-Acetic Acid Esterified with Waxy, Normal, and High-Amylose Maize Starches: Comparative Study on Colon-Targeted Delivery and Intestinal Health Impact.Nutrients. 2024 Oct 11;16(20):3446. doi: 10.3390/nu16203446. Nutrients. 2024. PMID: 39458442 Free PMC article.
-
The impact of traditional Chinese medicine and dietary compounds on modulating gut microbiota in hepatic fibrosis: A review.Heliyon. 2024 Sep 26;10(19):e38339. doi: 10.1016/j.heliyon.2024.e38339. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39391468 Free PMC article. Review.
-
Cross-talk between macrophages and gut microbiota in inflammatory bowel disease: a dynamic interplay influencing pathogenesis and therapy.Front Med (Lausanne). 2024 Sep 16;11:1457218. doi: 10.3389/fmed.2024.1457218. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39355844 Free PMC article. Review.
-
Metabolic mediators: microbial-derived metabolites as key regulators of anti-tumor immunity, immunotherapy, and chemotherapy.Front Immunol. 2024 Sep 16;15:1456030. doi: 10.3389/fimmu.2024.1456030. eCollection 2024. Front Immunol. 2024. PMID: 39351241 Free PMC article. Review.
-
The Impact of Yoyo Dieting and Resistant Starch on Weight Loss and Gut Microbiome in C57Bl/6 Mice.Nutrients. 2024 Sep 17;16(18):3138. doi: 10.3390/nu16183138. Nutrients. 2024. PMID: 39339738 Free PMC article.
References
-
- Aranha MM, Cortez-Pinto H, Costa A, da Silva IB, Camilo ME, de Moura MC, and Rodrigues CM (2008). Bile acid levels are increased in the liver of patients with steatohepatitis. Eur. J. Gastroenterol. Hepatol 20, 519–525. - PubMed
-
- Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, et al. (2012). Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61, 416–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

