Polycystic ovary syndrome: possible involvement of androgen-induced, chemerin-mediated ovarian recruitment of monocytes/macrophages
- PMID: 29688269
- PMCID: PMC6203875
- DOI: 10.1093/biolre/ioy096
Polycystic ovary syndrome: possible involvement of androgen-induced, chemerin-mediated ovarian recruitment of monocytes/macrophages
Abstract
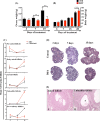
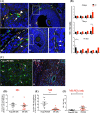
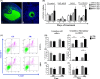
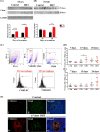
Polycystic ovary syndrome (PCOS) is a continuum of endocrine and reproductive disorders characterized by hyperandrogenism, antral follicle growth arrest, and chronic inflammation. Macrophages play key role in inflammation, and the balance between M1 (inflammatory) and M2 (anti-inflammatory) macrophages determines physiological/pathological outcomes. Here, we investigated if hyperandrogenism increases ovarian chemerin altering the balance of M1 and M2 macrophages and the granulosa cell death. Ovarian chemerin was upregulated by 5α-dihydrotestosterone (DHT) in lean and overweight rats; while increased serum chemerin levels were only evident in overweight rats, suggesting that the serum chemerin may be reflective of a systemic response and associated with obesity, whereas increased ovarian chemerin expression is a localized response independent of the metabolic status. DHT altered follicle dynamics while increased the M1: M2 macrophages ratio in antral and pre-ovulatory follicles. While ovarian M1 macrophages expressing chemokine-like receptor 1 (CMKLR1) were increased, CMKLR1+ monocytes, which migrated toward chemerin-rich environment, were markedly decreased after 15 days of DHT. Androgen-induced granulosa cell apoptosis was dependent on the presence of macrophages. In humans, chemerin levels in follicular fluid, but not in serum, were higher in lean PCOS patients compared to BMI-matched controls and were associated with increased M1: M2 ratio. Our results support the concept that in PCOS, hyperandrogenemia increases chemerin expression while promotes CMKLR1+ monocytes recruitment and deregulates the immunological niche of ovaries. This study established a new immunological perspective in PCOS at the ovarian level. Hyperandrogenism is associated with upregulation of chemerin and macrophage unbalance in the ovaries.
Figures



Similar articles
-
Chemerin, a novel regulator of follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome.Endocrinology. 2012 Nov;153(11):5600-11. doi: 10.1210/en.2012-1424. Epub 2012 Sep 4. Endocrinology. 2012. PMID: 22948218
-
Chemerin suppresses ovarian follicular development and its potential involvement in follicular arrest in rats treated chronically with dihydrotestosterone.Endocrinology. 2013 Aug;154(8):2912-23. doi: 10.1210/en.2013-1001. Epub 2013 May 21. Endocrinology. 2013. PMID: 23696570
-
Hyperandrogenic environment regulates the function of ovarian granulosa cells by modulating macrophage polarization in PCOS.Am J Reprod Immunol. 2024 May;91(5):e13854. doi: 10.1111/aji.13854. Am J Reprod Immunol. 2024. PMID: 38716832
-
[Research progress of chemerin in polycystic ovary syndrome].Sheng Li Xue Bao. 2024 Jun 25;76(3):429-437. Sheng Li Xue Bao. 2024. PMID: 38939937 Review. Chinese.
-
The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest.Hum Reprod Update. 2004 Mar-Apr;10(2):107-17. doi: 10.1093/humupd/dmh010. Hum Reprod Update. 2004. PMID: 15073141 Review.
Cited by
-
The role of the autonomic nervous system in polycystic ovary syndrome.Front Endocrinol (Lausanne). 2024 Jan 19;14:1295061. doi: 10.3389/fendo.2023.1295061. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38313837 Free PMC article. Review.
-
Neuropeptide Y regulates proliferation and apoptosis in granulosa cells in a follicular stage-dependent manner.J Ovarian Res. 2020 Jan 8;13(1):5. doi: 10.1186/s13048-019-0608-z. J Ovarian Res. 2020. PMID: 31915051 Free PMC article.
-
Preventive online and offline health management intervention in polycystic ovary syndrome.World J Clin Cases. 2022 Apr 6;10(10):3060-3068. doi: 10.12998/wjcc.v10.i10.3060. World J Clin Cases. 2022. PMID: 35647126 Free PMC article.
-
Neuropeptide Y directly reduced apoptosis of granulosa cells, and the expression of NPY and its receptors in PCOS subjects.J Ovarian Res. 2023 Aug 31;16(1):182. doi: 10.1186/s13048-023-01261-8. J Ovarian Res. 2023. PMID: 37653540 Free PMC article.
-
Immunological and Metabolic Causes of Infertility in Polycystic Ovary Syndrome.Biomedicines. 2023 May 28;11(6):1567. doi: 10.3390/biomedicines11061567. Biomedicines. 2023. PMID: 37371662 Free PMC article. Review.
References
-
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25(2):544–551. - PubMed
-
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81(1):19–25. - PubMed
-
- Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, Katsilambros N, Kreatsas G, Panidis D. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod 2006;21(6):1426–1431. - PubMed
-
- Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 2001; 86(6):2453–2455. - PubMed
-
- Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, Bellastella A, Carella C, Izzo A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol 2003; 101(6):1177–1182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

