TRPM2: a candidate therapeutic target for treating neurological diseases
- PMID: 29671419
- PMCID: PMC5943913
- DOI: 10.1038/aps.2018.31
TRPM2: a candidate therapeutic target for treating neurological diseases
Abstract
Transient receptor potential melastatin 2 (TRPM2) is a calcium (Ca2+)-permeable non-selective cation channel belonging to the TRP ion channel family. Oxidative stress-induced TRPM2 activation provokes aberrant intracellular Ca2+ accumulation and cell death in a variety of cell types, including neurons. Aberrant TRPM2 function has been implicated in several neurological disorders including ischemia/stroke, Alzheimer's disease, neuropathic pain, Parkinson's disease and bipolar disorder. In addition to research identifying a role for TRPM2 in disease, progress has been made in the identification of physiological functions of TRPM2 in the brain, including recent evidence that TRPM2 is necessary for the induction of N-methyl-D-aspartate (NMDA) receptor-dependent long-term depression, an important form of synaptic plasticity at glutamate synapses. Here, we summarize recent evidence on the role of TRPM2 in the central nervous system (CNS) in health and disease and discuss the potential therapeutic implications of targeting TRPM2. Collectively, these studies suggest that TRPM2 represents a prospective novel therapeutic target for neurological disorders.
Figures
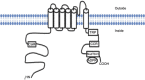
Similar articles
-
Medicinal chemistry perspective of TRPM2 channel inhibitors: where we are and where we might be heading?Drug Discov Today. 2020 Dec;25(12):2326-2334. doi: 10.1016/j.drudis.2020.09.039. Epub 2020 Oct 13. Drug Discov Today. 2020. PMID: 33065292 Review.
-
TRPM2: a multifunctional ion channel for calcium signalling.J Physiol. 2011 Apr 1;589(Pt 7):1515-25. doi: 10.1113/jphysiol.2010.201855. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135052 Free PMC article. Review.
-
Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium.Mol Neurobiol. 2017 Apr;54(3):2345-2360. doi: 10.1007/s12035-016-9727-3. Epub 2016 Mar 9. Mol Neurobiol. 2017. PMID: 26957303
-
Glia and TRPM2 Channels in Plasticity of Central Nervous System and Alzheimer's Diseases.Neural Plast. 2016;2016:1680905. doi: 10.1155/2016/1680905. Epub 2016 Jan 28. Neural Plast. 2016. PMID: 26942016 Free PMC article. Review.
-
Dependence of NMDA/GSK-3β mediated metaplasticity on TRPM2 channels at hippocampal CA3-CA1 synapses.Mol Brain. 2011 Dec 21;4:44. doi: 10.1186/1756-6606-4-44. Mol Brain. 2011. PMID: 22188973 Free PMC article.
Cited by
-
Chemical painful post-traumatic trigeminal neuropathy induced by dental bleaching: A case report.J Clin Exp Dent. 2024 Jan 1;16(1):e90-e95. doi: 10.4317/jced.60744. eCollection 2024 Jan. J Clin Exp Dent. 2024. PMID: 38314338 Free PMC article.
-
The identification of the key residues E829 and R845 involved in transient receptor potential melastatin 2 channel gating.Front Aging Neurosci. 2022 Oct 24;14:1033434. doi: 10.3389/fnagi.2022.1033434. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36353687 Free PMC article.
-
Targeting Transient Receptor Potential Melastatin-2 (TRPM2) Enhances Therapeutic Efficacy of Third Generation EGFR Inhibitors against EGFR Mutant Lung Cancer.Adv Sci (Weinh). 2024 Sep;11(35):e2310126. doi: 10.1002/advs.202310126. Epub 2024 Jul 23. Adv Sci (Weinh). 2024. PMID: 39044361 Free PMC article.
-
TRPM2 deficiency in mice protects against atherosclerosis by inhibiting TRPM2-CD36 inflammatory axis in macrophages.Nat Cardiovasc Res. 2022 Apr;1(4):344-360. doi: 10.1038/s44161-022-00027-7. Epub 2022 Mar 28. Nat Cardiovasc Res. 2022. PMID: 35445217 Free PMC article.
-
The transient receptor potential melastatin 2: a new therapeutical target for Parkinson's disease?Neural Regen Res. 2023 Aug;18(8):1652-1656. doi: 10.4103/1673-5374.360343. Neural Regen Res. 2023. PMID: 36751775 Free PMC article. Review.
References
-
- Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 1998; 54: 124–31. - PubMed
-
- Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 2006; 26: 159–78. - PubMed
-
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 1989; 2: 1313–23. - PubMed
-
- Clapham DE. SnapShot: mammalian TRP channels. Cell 2007; 129: 220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

