A Selective Extracellular Matrix Proteomics Approach Identifies Fibronectin Proteolysis by A Disintegrin-like and Metalloprotease Domain with Thrombospondin Type 1 Motifs (ADAMTS16) and Its Impact on Spheroid Morphogenesis
- PMID: 29669734
- PMCID: PMC6030725
- DOI: 10.1074/mcp.RA118.000676
A Selective Extracellular Matrix Proteomics Approach Identifies Fibronectin Proteolysis by A Disintegrin-like and Metalloprotease Domain with Thrombospondin Type 1 Motifs (ADAMTS16) and Its Impact on Spheroid Morphogenesis
Abstract
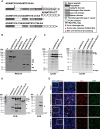
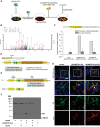
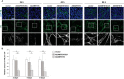
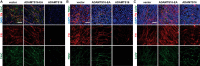
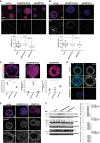
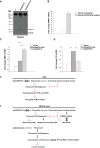
Secreted and cell-surface proteases are major mediators of extracellular matrix (ECM) turnover, but their mechanisms and regulatory impact are poorly understood. We developed a mass spectrometry approach using a cell-free ECM produced in vitro to identify fibronectin (FN) as a novel substrate of the secreted metalloprotease ADAMTS16. ADAMTS16 cleaves FN between its (I)5 and (I)6 modules, releasing the N-terminal 30 kDa heparin-binding domain essential for FN self-assembly. ADAMTS16 impairs FN fibrillogenesis as well as fibrillin-1 and tenascin-C assembly, thus inhibiting formation of a mature ECM by cultured fibroblasts. Furthermore ADAMTS16 has a marked morphogenetic impact on spheroid formation by renal tubule-derived MDCKI cells. The N-terminal FN domain released by ADAMTS16 up-regulates MMP3, which cleaves the (I)5-(I)6 linker of FN similar to ADAMTS16, therefore creating a proteolytic feed-forward mechanism. Thus, FN proteolysis not only regulates FN turnover, but also FN assembly, with potential long-term consequences for ECM assembly and morphogenesis.
Keywords: ADAMTS protease; Extracellular matrix*; Fibronectin; Metalloprotease; Post-translational modifications*; Proteases*; Protein Degradation*; Proteolysis*.
© 2018 Schnellmann et al.
Figures
Similar articles
-
A disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif 9 (ADAMTS9) regulates fibronectin fibrillogenesis and turnover.J Biol Chem. 2019 Jun 21;294(25):9924-9936. doi: 10.1074/jbc.RA118.006479. Epub 2019 May 13. J Biol Chem. 2019. PMID: 31085586 Free PMC article.
-
Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing.Mol Biol Cell. 2019 Aug 1;30(17):2218-2226. doi: 10.1091/mbc.E19-03-0140. Epub 2019 Jun 26. Mol Biol Cell. 2019. PMID: 31242089 Free PMC article.
-
Extracellular Matrix Disorganization Caused by ADAMTS16 Deficiency Leads to Bicuspid Aortic Valve With Raphe Formation.Circulation. 2024 Feb 20;149(8):605-626. doi: 10.1161/CIRCULATIONAHA.123.065458. Epub 2023 Nov 29. Circulation. 2024. PMID: 38018454
-
Profile of Matrix-Remodeling Proteinases in Osteoarthritis: Impact of Fibronectin.Cells. 2019 Dec 22;9(1):40. doi: 10.3390/cells9010040. Cells. 2019. PMID: 31877874 Free PMC article. Review.
-
Metalloproteinases: A parade of functions in matrix biology and an outlook for the future.Matrix Biol. 2015 May-Jul;44-46:1-6. doi: 10.1016/j.matbio.2015.04.005. Epub 2015 Apr 23. Matrix Biol. 2015. PMID: 25916966 Review.
Cited by
-
Secreted metalloproteases ADAMTS9 and ADAMTS20 have a non-canonical role in ciliary vesicle growth during ciliogenesis.Nat Commun. 2019 Feb 27;10(1):953. doi: 10.1038/s41467-019-08520-7. Nat Commun. 2019. PMID: 30814516 Free PMC article.
-
A disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif 9 (ADAMTS9) regulates fibronectin fibrillogenesis and turnover.J Biol Chem. 2019 Jun 21;294(25):9924-9936. doi: 10.1074/jbc.RA118.006479. Epub 2019 May 13. J Biol Chem. 2019. PMID: 31085586 Free PMC article.
-
ADAMTS18-fibronectin interaction regulates the morphology of liver sinusoidal endothelial cells.iScience. 2024 Jun 14;27(7):110273. doi: 10.1016/j.isci.2024.110273. eCollection 2024 Jul 19. iScience. 2024. PMID: 39040056 Free PMC article.
-
Fibronectin fragments generated by pancreatic trypsin act as endogenous inhibitors of pancreatic tumor growth.J Exp Clin Cancer Res. 2023 Aug 9;42(1):201. doi: 10.1186/s13046-023-02778-y. J Exp Clin Cancer Res. 2023. PMID: 37559126 Free PMC article.
-
ADAMTS Proteases: Their Multifaceted Role in the Regulation of Cancer Metastasis.Dis Res. 2024;4(1):40-52. doi: 10.54457/DR.202401004. Dis Res. 2024. PMID: 38948119 Free PMC article.
References
-
- Hynes R. O. (1990) Fibronectins. Springer- Verlag, New York
-
- Pankov R., and Yamada K. M. (2002) Fibronectin at a glance. J. Cell Sci. 115, 3861–3863 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

