Smoke-induced neuromuscular junction degeneration precedes the fibre type shift and atrophy in chronic obstructive pulmonary disease
- PMID: 29663403
- PMCID: PMC6046075
- DOI: 10.1113/JP275558
Smoke-induced neuromuscular junction degeneration precedes the fibre type shift and atrophy in chronic obstructive pulmonary disease
Abstract
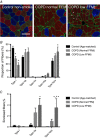
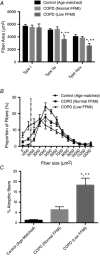
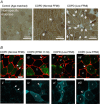
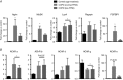
Key points: Chronic obstructive pulmonary disease (COPD) is largely caused by smoking, and patient limb muscle exhibits a fast fibre shift and atrophy. We show that this fast fibre shift is associated with type grouping, suggesting recurring cycles of denervation-reinnervation underlie the type shift. Compared to patients with normal fat-free mass index (FFMI), patients with low FFMI exhibited an exacerbated fibre type shift, marked accumulation of very small persistently denervated muscle fibres, and a blunted denervation-responsive transcript profile, suggesting failed denervation precipitates muscle atrophy in patients with low FFMI. Sixteen weeks of passive tobacco smoke exposure in mice caused neuromuscular junction degeneration, consistent with a key role for smoke exposure in initiating denervation in COPD.
Abstract: A neurological basis for the fast fibre shift and atrophy seen in limb muscle of patients with chronic obstructive pulmonary disease (COPD) has not been considered previously. The objective of our study was: (1) to determine if denervation contributes to fast fibre shift and muscle atrophy in COPD; and (2) to assess using a preclinical smoking mouse model whether chronic tobacco smoke (TS) exposure could initiate denervation by causing neuromuscular junction (NMJ) degeneration. Vastus lateralis muscle biopsies were obtained from severe COPD patients [n = 10 with low fat-free mass index (FFMI), 65 years; n = 15 normal FFMI, 65 years) and healthy age- and activity-matched non-smoker control subjects (CON; n = 11, 67 years), to evaluate morphological and transcriptional markers of denervation. To evaluate the potential for chronic TS exposure to initiate these changes, we examined NMJ morphology in male adult mice following 16 weeks of passive TS exposure. We observed a high proportion of grouped fast fibres and a denervation transcript profile in COPD patients, suggesting that motor unit remodelling drives the fast fibre type shift in COPD patient limb muscle. A further exacerbation of fast fibre grouping in patients with low FFMI, coupled with blunted reinnervation signals, accumulation of very small non-specific esterase hyperactive fibres and neural cell adhesion molecule-positive type I and type II fibres, suggests denervation-induced exhaustion of reinnervation contributes to muscle atrophy in COPD. Evidence from a smoking mouse model showed significant NMJ degeneration, suggesting that recurring denervation in COPD is probably caused by decades of chronic TS exposure.
Keywords: cachexia; denervation; muscle atrophy; sarcopenia; smoking mouse.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Conflict of interest statement
Jean Bourbeau receives grant funding from the (1) Research Chair COPD McGill University; (2) Research Institute of the McGill University Health Centre; (3) Research Chair COPD from GlaxoSmithKline; (4) CanCOLD consortium grant by Aerocrine, Almiral, AstraZeneca, Boehringer‐Ingleheim, GlaxoSmithKline, Novartis; and (v) Canadian Respiratory Research Network (CRRN) – Canadian Institutes of Health Research. R. Thomas Jagoe is a consultant for Immunotec Inc and related to this he has a patent for Compositions and Methods for Treatment of Muscle Wasting [US Patent Application 10/050,686 filed January 16 2003 (Harvard case 1829)]. None of the remaining authors declare any conflict of interest.
Figures
Similar articles
-
Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women.J Physiol. 2019 Oct;597(19):5009-5023. doi: 10.1113/JP278261. Epub 2019 Aug 21. J Physiol. 2019. PMID: 31368533
-
Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease.Eur Respir J. 2003 Aug;22(2):280-5. doi: 10.1183/09031936.03.00012803. Eur Respir J. 2003. PMID: 12952261
-
2D-DIGE proteomic analysis of vastus lateralis from COPD patients with low and normal fat free mass index and healthy controls.Respir Res. 2017 May 3;18(1):81. doi: 10.1186/s12931-017-0525-x. Respir Res. 2017. PMID: 28468631 Free PMC article.
-
Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis.Thorax. 2007 Nov;62(11):944-9. doi: 10.1136/thx.2007.078980. Epub 2007 May 25. Thorax. 2007. PMID: 17526675 Free PMC article. Review.
-
Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease.Int J Biochem Cell Biol. 2013 Oct;45(10):2245-56. doi: 10.1016/j.biocel.2013.06.015. Epub 2013 Jul 1. Int J Biochem Cell Biol. 2013. PMID: 23827718 Review.
Cited by
-
Skeletal Muscle Fiber Type in Hypoxia: Adaptation to High-Altitude Exposure and Under Conditions of Pathological Hypoxia.Front Physiol. 2018 Oct 12;9:1450. doi: 10.3389/fphys.2018.01450. eCollection 2018. Front Physiol. 2018. PMID: 30369887 Free PMC article. Review.
-
Update on the Etiology, Assessment, and Management of COPD Cachexia: Considerations for the Clinician.Int J Chron Obstruct Pulmon Dis. 2022 Nov 18;17:2957-2976. doi: 10.2147/COPD.S334228. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 36425061 Free PMC article. Review.
-
Mitochondrial Permeability Transition Causes Mitochondrial Reactive Oxygen Species- and Caspase 3-Dependent Atrophy of Single Adult Mouse Skeletal Muscle Fibers.Cells. 2021 Sep 29;10(10):2586. doi: 10.3390/cells10102586. Cells. 2021. PMID: 34685566 Free PMC article.
-
From amino-acid to disease: the effects of oxidation on actin-myosin interactions in muscle.J Muscle Res Cell Motil. 2023 Dec;44(4):225-254. doi: 10.1007/s10974-023-09658-0. Epub 2023 Oct 8. J Muscle Res Cell Motil. 2023. PMID: 37805961 Review.
-
Locomotor Muscles in COPD: The Rationale for Rehabilitative Exercise Training.Front Physiol. 2020 Jan 14;10:1590. doi: 10.3389/fphys.2019.01590. eCollection 2019. Front Physiol. 2020. PMID: 31992992 Free PMC article. Review.
References
-
- http://American Thoracic Society; http://American College of Chest Physicians. (2003). ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167, 211–277. - PubMed
-
- Baloh RH, Rakowicz W, Gardner R & Pestronk A (2007). Frequent atrophic groups with mixed‐type myofibers is distinctive to motor neuron syndromes. Muscle Nerve 36, 107–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

