The role of mitochondria in stem cell fate and aging
- PMID: 29654217
- PMCID: PMC5964648
- DOI: 10.1242/dev.143420
The role of mitochondria in stem cell fate and aging
Abstract
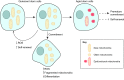
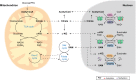
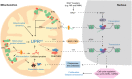
The importance of mitochondria in energy metabolism, signal transduction and aging in post-mitotic tissues has been well established. Recently, the crucial role of mitochondrial-linked signaling in stem cell function has come to light and the importance of mitochondria in mediating stem cell activity is becoming increasingly recognized. Despite the fact that many stem cells exhibit low mitochondrial content and a reliance on mitochondrial-independent glycolytic metabolism for energy, accumulating evidence has implicated the importance of mitochondrial function in stem cell activation, fate decisions and defense against senescence. In this Review, we discuss the recent advances that link mitochondrial metabolism, homeostasis, stress responses, and dynamics to stem cell function, particularly in the context of disease and aging. This Review will also highlight some recent progress in mitochondrial therapeutics that may present attractive strategies for improving stem cell function as a basis for regenerative medicine and healthy aging.
Keywords: Aging; Cell fate; Mitochondria; Stem cell.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
The Mitochondrial Basis of Aging.Mol Cell. 2016 Mar 3;61(5):654-666. doi: 10.1016/j.molcel.2016.01.028. Mol Cell. 2016. PMID: 26942670 Free PMC article. Review.
-
Reactive Oxygen Species and Mitochondrial Homeostasis as Regulators of Stem Cell Fate and Function.Antioxid Redox Signal. 2018 Jul 10;29(2):149-168. doi: 10.1089/ars.2017.7273. Epub 2017 Oct 26. Antioxid Redox Signal. 2018. PMID: 28708000 Review.
-
Mitochondrial unfolded protein response: a stress response with implications for fertility and reproductive aging.Fertil Steril. 2019 Feb;111(2):197-204. doi: 10.1016/j.fertnstert.2018.11.048. Fertil Steril. 2019. PMID: 30691623 Review.
-
Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection.Molecules. 2018 Jan 17;23(1):191. doi: 10.3390/molecules23010191. Molecules. 2018. PMID: 29342113 Free PMC article. Review.
-
Mitochondrial plasticity in cell fate regulation.J Biol Chem. 2019 Sep 20;294(38):13852-13863. doi: 10.1074/jbc.REV118.000828. Epub 2019 Aug 5. J Biol Chem. 2019. PMID: 31383739 Free PMC article. Review.
Cited by
-
Bioenergetics matter to metabolic health-from a fat progenitor view.Cell Stem Cell. 2021 Apr 1;28(4):589-591. doi: 10.1016/j.stem.2021.03.008. Cell Stem Cell. 2021. PMID: 33798417 Free PMC article.
-
Exosome-shuttled mitochondrial transcription factor A mRNA promotes the osteogenesis of dental pulp stem cells through mitochondrial oxidative phosphorylation activation.Cell Prolif. 2022 Dec;55(12):e13324. doi: 10.1111/cpr.13324. Epub 2022 Aug 26. Cell Prolif. 2022. PMID: 36054692 Free PMC article.
-
Human retinal organoids with an OPA1 mutation are defective in retinal ganglion cell differentiation and function.Stem Cell Reports. 2024 Jan 9;19(1):68-83. doi: 10.1016/j.stemcr.2023.11.004. Epub 2023 Dec 14. Stem Cell Reports. 2024. PMID: 38101398 Free PMC article.
-
Chewing the Fat with Microbes: Lipid Crosstalk in the Gut.Nutrients. 2022 Jan 28;14(3):573. doi: 10.3390/nu14030573. Nutrients. 2022. PMID: 35276931 Free PMC article. Review.
-
Lipid droplet dynamics regulate adult muscle stem cell fate.Cell Rep. 2022 Jan 18;38(3):110267. doi: 10.1016/j.celrep.2021.110267. Cell Rep. 2022. PMID: 35045287 Free PMC article.
References
-
- Ahlqvist K. J., Hämäläinen R. H., Yatsuga S., Uutela M., Terzioglu M., Götz A., Forsstrom S., Salven P., Angers-Loustau A., Kopra O. H. et al. (2012). Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. 15, 100-109. 10.1016/j.cmet.2011.11.012 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

