n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease
- PMID: 29652551
- PMCID: PMC5952353
- DOI: 10.1056/NEJMoa1709691
n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease
Abstract
Background: Dry eye disease is a common chronic condition that is characterized by ocular discomfort and visual disturbances that decrease quality of life. Many clinicians recommend the use of supplements of n-3 fatty acids (often called omega-3 fatty acids) to relieve symptoms.
Methods: In a multicenter, double-blind clinical trial, we randomly assigned patients with moderate-to-severe dry eye disease to receive a daily oral dose of 3000 mg of fish-derived n-3 eicosapentaenoic and docosahexaenoic acids (active supplement group) or an olive oil placebo (placebo group). The primary outcome was the mean change from baseline in the score on the Ocular Surface Disease Index (OSDI; scores range from 0 to 100, with higher scores indicating greater symptom severity), which was based on the mean of scores obtained at 6 and 12 months. Secondary outcomes included mean changes per eye in the conjunctival staining score (ranging from 0 to 6) and the corneal staining score (ranging from 0 to 15), with higher scores indicating more severe damage to the ocular surface, as well as mean changes in the tear break-up time (seconds between a blink and gaps in the tear film) and the result on Schirmer's test (length of wetting of paper strips placed on the lower eyelid), with lower values indicating more severe signs.
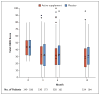
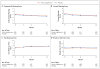
Results: A total of 349 patients were assigned to the active supplement group and 186 to the placebo group; the primary analysis included 329 and 170 patients, respectively. The mean change in the OSDI score was not significantly different between the active supplement group and the placebo group (-13.9 points and -12.5 points, respectively; mean difference in change after imputation of missing data, -1.9 points; 95% confidence interval [CI], -5.0 to 1.1; P=0.21). This result was consistent across prespecified subgroups. There were no significant differences between the active supplement group and the placebo group in mean changes from baseline in the conjunctival staining score (mean difference in change, 0.0 points; 95% CI, -0.2 to 0.1), corneal staining score (0.1 point; 95% CI, -0.2 to 0.4), tear break-up time (0.2 seconds; 95% CI, -0.1 to 0.5), and result on Schirmer's test (0.0 mm; 95% CI, -0.8 to 0.9). At 12 months, the rate of adherence to treatment in the active supplement group was 85.2%, according to the level of n-3 fatty acids in red cells. Rates of adverse events were similar in the two trial groups.
Conclusions: Among patients with dry eye disease, those who were randomly assigned to receive supplements containing 3000 mg of n-3 fatty acids for 12 months did not have significantly better outcomes than those who were assigned to receive placebo. (Funded by the National Eye Institute, National Institutes of Health; DREAM ClinicalTrials.gov number, NCT02128763 .).
Figures
Comment in
-
n-3 Fatty Acid Supplementation and Dry Eye Disease.N Engl J Med. 2018 Aug 16;379(7):690-691. doi: 10.1056/NEJMc1807693. N Engl J Med. 2018. PMID: 30110584 No abstract available.
-
n-3 Fatty Acid Supplementation and Dry Eye Disease.N Engl J Med. 2018 Aug 16;379(7):691. doi: 10.1056/NEJMc1807693. N Engl J Med. 2018. PMID: 30124262 No abstract available.
-
Why DREAM should make you think twice about recommending Omega-3 supplements.Ocul Surf. 2019 Oct;17(4):617-618. doi: 10.1016/j.jtos.2019.08.003. Epub 2019 Aug 12. Ocul Surf. 2019. PMID: 31415816 No abstract available.
Similar articles
-
Short-term consumption of oral omega-3 and dry eye syndrome.Ophthalmology. 2013 Nov;120(11):2191-6. doi: 10.1016/j.ophtha.2013.04.006. Epub 2013 May 1. Ophthalmology. 2013. PMID: 23642375 Clinical Trial.
-
Effect of a Novel Omega-3 and Omega-6 Fatty Acid Supplement on Dry Eye Disease: A 3-month Randomized Controlled Trial.Optom Vis Sci. 2022 Jan 1;99(1):67-75. doi: 10.1097/OPX.0000000000001826. Optom Vis Sci. 2022. PMID: 34882608 Clinical Trial.
-
The Dry Eye Assessment and Management (DREAM) extension study - A randomized clinical trial of withdrawal of supplementation with omega-3 fatty acid in patients with dry eye disease.Ocul Surf. 2020 Jan;18(1):47-55. doi: 10.1016/j.jtos.2019.08.002. Epub 2019 Aug 16. Ocul Surf. 2020. PMID: 31425752 Free PMC article. Clinical Trial.
-
EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials.J Am Coll Nutr. 2009 Oct;28(5):525-42. doi: 10.1080/07315724.2009.10719785. J Am Coll Nutr. 2009. PMID: 20439549 Review.
-
Punctal occlusion for dry eye syndrome.Cochrane Database Syst Rev. 2017 Jun 26;6(6):CD006775. doi: 10.1002/14651858.CD006775.pub3. Cochrane Database Syst Rev. 2017. PMID: 28649802 Free PMC article. Review.
Cited by
-
Association of meibomian gland morphology with symptoms and signs of dry eye disease in the Dry Eye Assessment and Management (DREAM) study.Ocul Surf. 2020 Oct;18(4):761-769. doi: 10.1016/j.jtos.2020.07.014. Epub 2020 Aug 25. Ocul Surf. 2020. PMID: 32858234 Free PMC article.
-
A Novel Integrated Active Herbal Formulation Ameliorates Dry Eye Syndrome by Inhibiting Inflammation and Oxidative Stress and Enhancing Glycosylated Phosphoproteins in Rats.Pharmaceuticals (Basel). 2020 Oct 7;13(10):295. doi: 10.3390/ph13100295. Pharmaceuticals (Basel). 2020. PMID: 33036453 Free PMC article.
-
Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape.Ophthalmol Ther. 2023 Jun;12(3):1397-1418. doi: 10.1007/s40123-023-00669-1. Epub 2023 Mar 1. Ophthalmol Ther. 2023. PMID: 36856980 Free PMC article. Review.
-
Managing Dry Eye Disease and Facilitating Realistic Patient Expectations: A Review and Appraisal of Current Therapies.Clin Ophthalmol. 2020 Jan 14;14:119-126. doi: 10.2147/OPTH.S228838. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32021076 Free PMC article. Review.
-
The potential of lipid mediator networks as ocular surface therapeutics and biomarkers.Ocul Surf. 2021 Jan;19:104-114. doi: 10.1016/j.jtos.2020.04.008. Epub 2020 Apr 29. Ocul Surf. 2021. PMID: 32360792 Free PMC article. Review.
References
-
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. - PubMed
-
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65. - PubMed
-
- Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–87. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
