The cell biology of systemic insulin function
- PMID: 29622564
- PMCID: PMC6028526
- DOI: 10.1083/jcb.201802095
The cell biology of systemic insulin function
Abstract
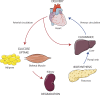
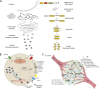
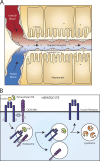
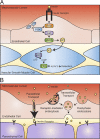
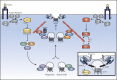
Insulin is the paramount anabolic hormone, promoting carbon energy deposition in the body. Its synthesis, quality control, delivery, and action are exquisitely regulated by highly orchestrated intracellular mechanisms in different organs or "stations" of its bodily journey. In this Beyond the Cell review, we focus on these five stages of the journey of insulin through the body and the captivating cell biology that underlies the interaction of insulin with each organ. We first analyze insulin's biosynthesis in and export from the β-cells of the pancreas. Next, we focus on its first pass and partial clearance in the liver with its temporality and periodicity linked to secretion. Continuing the journey, we briefly describe insulin's action on the blood vasculature and its still-debated mechanisms of exit from the capillary beds. Once in the parenchymal interstitium of muscle and adipose tissue, insulin promotes glucose uptake into myofibers and adipocytes, and we elaborate on the intricate signaling and vesicle traffic mechanisms that underlie this fundamental function. Finally, we touch upon the renal degradation of insulin to end its action. Cellular discernment of insulin's availability and action should prove critical to understanding its pivotal physiological functions and how their failure leads to diabetes.
© 2018 Tokarz et al.
Figures
Similar articles
-
Active role for the vasculature in the delivery of insulin to skeletal muscle.Clin Exp Pharmacol Physiol. 2005 Apr;32(4):302-7. doi: 10.1111/j.1440-1681.2005.04188.x. Clin Exp Pharmacol Physiol. 2005. PMID: 15810996 Review.
-
Trans-fatty acids aggravate anabolic steroid-induced metabolic disturbances and differential gene expression in muscle, pancreas and adipose tissue.Life Sci. 2019 Sep 1;232:116603. doi: 10.1016/j.lfs.2019.116603. Epub 2019 Jun 27. Life Sci. 2019. PMID: 31254587
-
[Insulin signaling and pathophysiology of type 2 diabetes mellitus].Nihon Rinsho. 2006 Jul;64(7):1381-9. Nihon Rinsho. 2006. PMID: 16838661 Review. Japanese.
-
[Relative importance of insulin actin in insulin's target tissues].Nihon Rinsho. 2000 Feb;58(2):291-6. Nihon Rinsho. 2000. PMID: 10707547 Review. Japanese.
-
MiR-155 Enhances Insulin Sensitivity by Coordinated Regulation of Multiple Genes in Mice.PLoS Genet. 2016 Oct 6;12(10):e1006308. doi: 10.1371/journal.pgen.1006308. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27711113 Free PMC article.
Cited by
-
Effects of dietary supplementation in treatment and control of progression and complications of insulin-dependent diabetes mellitus: a systematic review with meta-analyses of randomized clinical trials.Braz J Med Biol Res. 2024 Aug 23;57:e13649. doi: 10.1590/1414-431X2024e13649. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 39194033 Free PMC article.
-
Bariatric surgery for diabetic comorbidities: A focus on hepatic, cardiac and renal fibrosis.Front Pharmacol. 2022 Oct 21;13:1016635. doi: 10.3389/fphar.2022.1016635. eCollection 2022. Front Pharmacol. 2022. PMID: 36339532 Free PMC article. Review.
-
Combination of melt-electrospun poly-ε-caprolactone scaffolds and hepatocyte-like cells from footprint-free hiPSCs to create 3D biohybrid constructs for liver tissue engineering.Sci Rep. 2023 Dec 13;13(1):22174. doi: 10.1038/s41598-023-49117-x. Sci Rep. 2023. PMID: 38092880 Free PMC article.
-
β-cell function in black South African women: exploratory associations with insulin clearance, visceral and ectopic fat.Endocr Connect. 2021 May 19;10(5):550-560. doi: 10.1530/EC-21-0153. Endocr Connect. 2021. PMID: 33884957 Free PMC article.
-
Trace Amine-Associated Receptors and Monoamine-Mediated Regulation of Insulin Secretion in Pancreatic Islets.Biomolecules. 2023 Nov 5;13(11):1618. doi: 10.3390/biom13111618. Biomolecules. 2023. PMID: 38002300 Free PMC article. Review.
References
-
- Ader M., Stefanovski D., Kim S.P., Richey J.M., Ionut V., Catalano K.J., Hucking K., Ellmerer M., Van Citters G., Hsu I.R., et al. . 2014. Hepatic insulin clearance is the primary determinant of insulin sensitivity in the normal dog. Obesity (Silver Spring). 22:1238–1245. 10.1002/oby.20625 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

