Preclinical efficacy studies in investigator brochures: Do they enable risk-benefit assessment?
- PMID: 29621228
- PMCID: PMC5886385
- DOI: 10.1371/journal.pbio.2004879
Preclinical efficacy studies in investigator brochures: Do they enable risk-benefit assessment?
Abstract
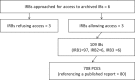
Human protection policies require favorable risk-benefit judgments prior to launch of clinical trials. For phase I and II trials, evidence for such judgment often stems from preclinical efficacy studies (PCESs). We undertook a systematic investigation of application materials (investigator brochures [IBs]) presented for ethics review for phase I and II trials to assess the content and properties of PCESs contained in them. Using a sample of 109 IBs most recently approved at 3 institutional review boards based at German Medical Faculties between the years 2010-2016, we identified 708 unique PCESs. We then rated all identified PCESs for their reporting on study elements that help to address validity threats, whether they referenced published reports, and the direction of their results. Altogether, the 109 IBs reported on 708 PCESs. Less than 5% of all PCESs described elements essential for reducing validity threats such as randomization, sample size calculation, and blinded outcome assessment. For most PCESs (89%), no reference to a published report was provided. Only 6% of all PCESs reported an outcome demonstrating no effect. For the majority of IBs (82%), all PCESs were described as reporting positive findings. Our results show that most IBs for phase I/II studies did not allow evaluators to systematically appraise the strength of the supporting preclinical findings. The very rare reporting of PCESs that demonstrated no effect raises concerns about potential design or reporting biases. Poor PCES design and reporting thwart risk-benefit evaluation during ethical review of phase I/II studies.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: JK serves in a remunerative capacity on the data safety monitoring board of an early-phase trial being pursued by Dimension Therapeutics. JK is part of the Editorial Board of the Meta-Research section of
Figures
Similar articles
-
Investigator brochures for phase I/II trials lack information on the robustness of preclinical safety studies.Br J Clin Pharmacol. 2021 Jul;87(7):2723-2731. doi: 10.1111/bcp.14615. Epub 2020 Nov 20. Br J Clin Pharmacol. 2021. PMID: 33068032
-
Reporting of prior clinical studies in Investigator's Brochures did not adhere to the basic principles of evidence synthesis: a cross-sectional study.J Clin Epidemiol. 2021 Feb;130:87-95. doi: 10.1016/j.jclinepi.2020.09.022. Epub 2020 Sep 28. J Clin Epidemiol. 2021. PMID: 32991993
-
Preclinical efficacy in investigator's brochures: Stakeholders' views on measures to improve completeness and robustness.Br J Clin Pharmacol. 2023 Jan;89(1):340-350. doi: 10.1111/bcp.15503. Epub 2022 Aug 31. Br J Clin Pharmacol. 2023. PMID: 35986927 Review.
-
Assessing risk/benefit for trials using preclinical evidence: a proposal.J Med Ethics. 2016 Jan;42(1):50-3. doi: 10.1136/medethics-2015-102882. Epub 2015 Oct 13. J Med Ethics. 2016. PMID: 26463620 Free PMC article.
-
Proposal for a "phase II" multicenter trial model for preclinical new antiepilepsy therapy development.Epilepsia. 2013 Aug;54 Suppl 4:70-4. doi: 10.1111/epi.12300. Epilepsia. 2013. PMID: 23909855 Review.
Cited by
-
Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0.PLoS Biol. 2020 Jul 14;18(7):e3000411. doi: 10.1371/journal.pbio.3000411. eCollection 2020 Jul. PLoS Biol. 2020. PMID: 32663221 Free PMC article.
-
A standardised framework to identify optimal animal models for efficacy assessment in drug development.PLoS One. 2019 Jun 13;14(6):e0218014. doi: 10.1371/journal.pone.0218014. eCollection 2019. PLoS One. 2019. PMID: 31194784 Free PMC article.
-
Initial heritable genome editing: mapping a responsible pathway from basic research to the clinic.Med Health Care Philos. 2023 Mar;26(1):21-35. doi: 10.1007/s11019-022-10115-x. Epub 2022 Nov 22. Med Health Care Philos. 2023. PMID: 36414813 Free PMC article.
-
[Transparency in clinical research: What contribution does the new EU Regulation 536/2014 make?].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2023 Jan;66(1):52-59. doi: 10.1007/s00103-022-03631-x. Epub 2022 Dec 13. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2023. PMID: 36512076 Free PMC article. Review. German.
-
Synthesizing regulatory guidance for demonstrating preclinical efficacy and translating promising cell therapies to early phase clinical trials: a scoping review.BMC Med. 2024 Oct 23;22(1):487. doi: 10.1186/s12916-024-03690-8. BMC Med. 2024. PMID: 39443960 Free PMC article.
References
-
- Kimmelman J. A theoretical framework for early human studies: uncertainty, intervention ensembles, and boundaries. Trials. 2012;13:173 doi: 10.1186/1745-6215-13-173 ; PubMed Central PMCID: PMC3551836. - DOI - PMC - PubMed
-
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8(3):e1000344 doi: 10.1371/journal.pbio.1000344 ; PubMed Central PMCID: PMC2846857. - DOI - PMC - PubMed
-
- Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483(7391):531–3. doi: 10.1038/483531a . - DOI - PubMed
-
- ter Riet G, Korevaar DA, Leenaars M, Sterk PJ, Van Noorden CJ, Bouter LM, et al. Publication bias in laboratory animal research: a survey on magnitude, drivers, consequences and potential solutions. PLoS ONE. 2012;7(9):e43404 doi: 10.1371/journal.pone.0043404 ; PubMed Central PMCID: PMC3434185. - DOI - PMC - PubMed
-
- Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One. 2009;4(11):e7824 doi: 10.1371/journal.pone.0007824 ; PubMed Central PMCID: PMC2779358. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical