Exosomal Tat protein activates latent HIV-1 in primary, resting CD4+ T lymphocytes
- PMID: 29618654
- PMCID: PMC5928859
- DOI: 10.1172/jci.insight.95676
Exosomal Tat protein activates latent HIV-1 in primary, resting CD4+ T lymphocytes
Abstract
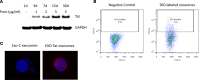
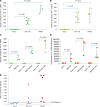
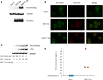
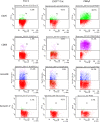
Replication competent HIV-1 persists in a subpopulation of CD4+ T lymphocytes despite prolonged antiretroviral treatment. This residual reservoir of infected cells harbors transcriptionally silent provirus capable of reigniting productive infection upon discontinuation of antiretroviral therapy. Certain classes of drugs can activate latent virus but not at levels that lead to reductions in HIV-1 reservoir size in vivo. Here, we show the utility of CD4+ receptor targeting exosomes as an HIV-1 latency reversal agent (LRA). We engineered human cellular exosomes to express HIV-1 Tat, a protein that is a potent transactivator of viral transcription. Preparations of exosomal Tat-activated HIV-1 in primary, resting CD4+ T lymphocytes isolated from antiretroviral-treated individuals with prolonged periods of viral suppression and led to the production of replication competent HIV-1. Furthermore, exosomal Tat increased the potency of selected LRA by over 30-fold in terms of HIV-1 mRNA expression, thereby establishing it as a potentially new class of biologic product with possible combinatorial utility in targeting latent HIV-1.
Keywords: AIDS/HIV; Drug therapy; Infectious disease; Molecular biology; T cells.
Conflict of interest statement
Figures




Similar articles
-
Maraviroc Is Associated with Latent HIV-1 Reactivation through NF-κB Activation in Resting CD4+ T Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy.J Virol. 2018 Apr 13;92(9):e01931-17. doi: 10.1128/JVI.01931-17. Print 2018 May 1. J Virol. 2018. PMID: 29444937 Free PMC article.
-
Regulation of human immunodeficiency virus-1 latency and its reactivation.Bull Mem Acad R Med Belg. 2008;163(6):355-64; discussion 364-5. Bull Mem Acad R Med Belg. 2008. PMID: 19445107
-
A stable latent reservoir for HIV-1 in resting CD4(+) T lymphocytes in infected children.J Clin Invest. 2000 Apr;105(7):995-1003. doi: 10.1172/JCI9006. J Clin Invest. 2000. PMID: 10749578 Free PMC article.
-
Therapeutic Approaches to Eradicate Latent HIV-1 in Resting CD4+ T Cells.Curr Top Med Chem. 2016;16(10):1191-7. doi: 10.2174/1568026615666150901114138. Curr Top Med Chem. 2016. PMID: 26324046 Review.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
Cited by
-
HIV latency reversing agents act through Tat post translational modifications.Retrovirology. 2018 May 11;15(1):36. doi: 10.1186/s12977-018-0421-6. Retrovirology. 2018. PMID: 29751762 Free PMC article.
-
Human Immunodeficiency Virus 1 (HIV-1): Viral Latency, the Reservoir, and the Cure.Yale J Biol Med. 2020 Sep 30;93(4):549-560. eCollection 2020 Sep. Yale J Biol Med. 2020. PMID: 33005119 Free PMC article. Review.
-
A Review of Current Strategies Towards the Elimination of Latent HIV-1 and Subsequent HIV-1 Cure.Curr HIV Res. 2021;19(1):14-26. doi: 10.2174/1570162X18999200819172009. Curr HIV Res. 2021. PMID: 32819259 Free PMC article.
-
Considerations for extracellular vesicle and lipoprotein interactions in cell culture assays.J Extracell Vesicles. 2022 Apr;11(4):e12202. doi: 10.1002/jev2.12202. J Extracell Vesicles. 2022. PMID: 35362268 Free PMC article.
-
Development of miRNA-based therapeutic approaches for cancer patients.Cancer Sci. 2019 Apr;110(4):1140-1147. doi: 10.1111/cas.13965. Epub 2019 Feb 26. Cancer Sci. 2019. PMID: 30729639 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

