Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo
- PMID: 29593039
- PMCID: PMC5974478
- DOI: 10.1128/JVI.00235-18
Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo
Erratum in
-
Correction for Garrido et al., "Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo".J Virol. 2020 May 18;94(11):e00223-20. doi: 10.1128/JVI.00223-20. Print 2020 May 18. J Virol. 2020. PMID: 32423966 Free PMC article. No abstract available.
Abstract
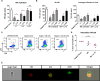
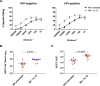
Current efforts toward human immunodeficiency virus (HIV) eradication include approaches to augment immune recognition and elimination of persistently infected cells following latency reversal. Natural killer (NK) cells, the main effectors of the innate immune system, recognize and clear targets using different mechanisms than CD8+ T cells, offering an alternative or complementary approach for HIV clearance strategies. We assessed the impact of interleukin 15 (IL-15) treatment on NK cell function and the potential for stimulated NK cells to clear the HIV reservoir. We measured NK cell receptor expression, antibody-dependent cell-mediated cytotoxicity (ADCC), cytotoxicity, interferon gamma (IFN-γ) production, and antiviral activity in autologous HIV replication systems. All NK cell functions were uniformly improved by IL-15, and, more importantly, IL-15-treated NK cells were able to clear latently HIV-infected cells after exposure to vorinostat, a clinically relevant latency-reversing agent. We also demonstrate that NK cells from HIV-infected individuals aviremic on antiretroviral therapy can be efficiently stimulated with IL-15. Our work opens a promising line of investigation leading to future immunotherapies to clear persistent HIV infection using NK cells.IMPORTANCE In the search for an HIV cure, strategies to enhance immune function to allow recognition and clearance of HIV-infected cells following latency reversal are being evaluated. Natural killer (NK) cells possess characteristics that can be exploited for immunotherapy against persistent HIV infection. We demonstrate that NK cells from HIV-positive donors can be strongly stimulated with IL-15, improving their antiviral and cytotoxic potential and, more importantly, clearing HIV-infected cells after latency reversal with a clinically relevant drug. Our results encourage further investigation to design NK cell-based immunotherapies to achieve HIV eradication.
Keywords: ADCC; HIV; HIV eradication; IL-15; NK cell; SAHA; VOR; human immunodeficiency virus; immune function; immunotherapy; interleukins; kick and kill; latency reversal; natural killer cells; shock and kill; vorinostat.
Copyright © 2018 American Society for Microbiology.
Figures



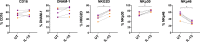
Similar articles
-
TLR1/2 Agonist Enhances Reversal of HIV-1 Latency and Promotes NK Cell-Induced Suppression of HIV-1-Infected Autologous CD4+ T Cells.J Virol. 2021 Aug 10;95(17):e0081621. doi: 10.1128/JVI.00816-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34133900 Free PMC article.
-
A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells.J Virol. 2016 Apr 14;90(9):4441-4453. doi: 10.1128/JVI.00222-16. Print 2016 May. J Virol. 2016. PMID: 26889036 Free PMC article.
-
The HIV Latency Reversal Agent HODHBt Enhances NK Cell Effector and Memory-Like Functions by Increasing Interleukin-15-Mediated STAT Activation.J Virol. 2022 Aug 10;96(15):e0037222. doi: 10.1128/jvi.00372-22. Epub 2022 Jul 14. J Virol. 2022. PMID: 35867565 Free PMC article.
-
Potential of the NKG2D/NKG2DL Axis in NK Cell-Mediated Clearance of the HIV-1 Reservoir.Int J Mol Sci. 2019 Sep 11;20(18):4490. doi: 10.3390/ijms20184490. Int J Mol Sci. 2019. PMID: 31514330 Free PMC article. Review.
-
Therapeutic Targeting of HIV Reservoirs: How to Give T Cells a New Direction.Front Immunol. 2018 Dec 4;9:2861. doi: 10.3389/fimmu.2018.02861. eCollection 2018. Front Immunol. 2018. PMID: 30564246 Free PMC article. Review.
Cited by
-
Harnessing Natural Killer Cell Innate and Adaptive Traits in HIV Infection.Front Cell Infect Microbiol. 2020 Aug 4;10:395. doi: 10.3389/fcimb.2020.00395. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32850493 Free PMC article. Review.
-
Safety and Virologic Impact of Haploidentical NK Cells Plus Interleukin 2 or N-803 in HIV Infection.J Infect Dis. 2024 May 15;229(5):1256-1265. doi: 10.1093/infdis/jiad578. J Infect Dis. 2024. PMID: 38207119 Free PMC article. Clinical Trial.
-
Immune targeting of HIV-1 reservoir cells: a path to elimination strategies and cure.Nat Rev Microbiol. 2024 Jun;22(6):328-344. doi: 10.1038/s41579-024-01010-8. Epub 2024 Feb 9. Nat Rev Microbiol. 2024. PMID: 38337034 Free PMC article. Review.
-
Insights into the HIV Latency and the Role of Cytokines.Pathogens. 2019 Sep 4;8(3):137. doi: 10.3390/pathogens8030137. Pathogens. 2019. PMID: 31487807 Free PMC article. Review.
-
Altered Cervical Mucosal Gene Expression and Lower Interleukin 15 Levels in Women With Schistosoma haematobium Infection but Not in Women With Schistosoma mansoni Infection.J Infect Dis. 2019 May 5;219(11):1777-1785. doi: 10.1093/infdis/jiy742. J Infect Dis. 2019. PMID: 30590736 Free PMC article.
References
-
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482–485. doi:10.1038/nature11286. - DOI - PMC - PubMed
-
- Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Ostergaard L, Tolstrup M. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi:10.1371/journal.ppat.1005142. - DOI - PMC - PubMed
-
- Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Ostergaard L, Sogaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi:10.1016/S2352-3018(14)70014-1. - DOI - PubMed
-
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi:10.1038/nature05115. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

