Human cytomegalovirus reprogrammes haematopoietic progenitor cells into immunosuppressive monocytes to achieve latency
- PMID: 29588542
- PMCID: PMC6537872
- DOI: 10.1038/s41564-018-0131-9
Human cytomegalovirus reprogrammes haematopoietic progenitor cells into immunosuppressive monocytes to achieve latency
Abstract
The precise cell type hosting latent human cytomegalovirus (HCMV) remains elusive. Here, we report that HCMV reprogrammes human haematopoietic progenitor cells (HPCs) into a unique monocyte subset to achieve latency. Unlike conventional monocytes, this monocyte subset possesses higher levels of B7-H4, IL-10 and inducible nitric oxide synthase (iNOS), a longer lifespan and strong immunosuppressive capacity. Cell sorting of peripheral blood from latently infected human donors confirms that only this monocyte subset, representing less than 0.1% of peripheral mononuclear cells, is HCMV genome-positive but immediate-early-negative. Mechanistic studies demonstrate that HCMV promotes the differentiation of HPCs into this monocyte subset by activating cellular signal transducer and activator of transcription 3 (STAT3). In turn, this monocyte subset generates a high level of nitric oxide (NO) to silence HCMV immediate-early transcription and promote viral latency. By contrast, the US28-knockout HCMV mutant, which is incapable of activating STAT3, fails to reprogramme the HPCs and achieve latency. Our findings reveal that via activating the STAT3-iNOS-NO axis, HCMV differentiates human HPCs into a longevous, immunosuppressive monocyte subset for viral latency.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



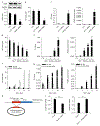
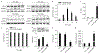

Comment in
-
Viral programming of progenitor cell commitment.Nat Microbiol. 2018 Apr;3(4):398-399. doi: 10.1038/s41564-018-0136-4. Nat Microbiol. 2018. PMID: 29588537 No abstract available.
Similar articles
-
Single cell analysis reveals human cytomegalovirus drives latently infected cells towards an anergic-like monocyte state.Elife. 2020 Jan 22;9:e52168. doi: 10.7554/eLife.52168. Elife. 2020. PMID: 31967545 Free PMC article.
-
Human Cytomegalovirus US28 Ligand Binding Activity Is Required for Latency in CD34+ Hematopoietic Progenitor Cells and Humanized NSG Mice.mBio. 2019 Aug 20;10(4):e01889-19. doi: 10.1128/mBio.01889-19. mBio. 2019. PMID: 31431555 Free PMC article.
-
Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro.J Virol. 2014 Aug;88(16):9391-405. doi: 10.1128/JVI.00934-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920803 Free PMC article.
-
Human cytomegalovirus infection of human hematopoietic progenitor cells.Leuk Lymphoma. 1999 Mar;33(1-2):1-13. doi: 10.3109/10428199909093720. Leuk Lymphoma. 1999. PMID: 10194116 Review.
-
Human cytomegalovirus reactivation in bone-marrow-derived granulocyte/monocyte progenitor cells and mature monocytes.Intervirology. 1999;42(5-6):308-13. doi: 10.1159/000053965. Intervirology. 1999. PMID: 10702711 Review.
Cited by
-
Human Cytomegalovirus Primary Infection and Reactivation: Insights From Virion-Carried Molecules.Front Microbiol. 2020 Jul 14;11:1511. doi: 10.3389/fmicb.2020.01511. eCollection 2020. Front Microbiol. 2020. PMID: 32765441 Free PMC article. Review.
-
Epigenetic reprogramming of host and viral genes by Human Cytomegalovirus infection in Kasumi-3 myeloid progenitor cells at early times post-infection.J Virol. 2021 May 10;95(11):e00183-21. doi: 10.1128/JVI.00183-21. Epub 2021 Mar 17. J Virol. 2021. PMID: 33731453 Free PMC article.
-
A Broad Application of CRISPR Cas9 in Infectious Diseases of Central Nervous System.J Neuroimmune Pharmacol. 2019 Dec;14(4):578-594. doi: 10.1007/s11481-019-09878-7. Epub 2019 Sep 11. J Neuroimmune Pharmacol. 2019. PMID: 31512166 Free PMC article. Review.
-
Herpesviral Latency-Common Themes.Pathogens. 2020 Feb 15;9(2):125. doi: 10.3390/pathogens9020125. Pathogens. 2020. PMID: 32075270 Free PMC article. Review.
-
Methods for Studying the Function of Cytomegalovirus GPCRs.Methods Mol Biol. 2021;2244:159-197. doi: 10.1007/978-1-0716-1111-1_9. Methods Mol Biol. 2021. PMID: 33555587
References
-
- Sinclair J & Sissons P Latency and reactivation of human cytomegalovirus. J. Gen. Virol 87, 1763–1779 (2006). - PubMed
-
- Sissons JG & Carmichael AJ Clinical aspects and management of cytomegalovirus infection. J. Infect 44, 78–83 (2002). - PubMed
-
- Reeves MB & Sinclair JH Analysis of latent viral gene expression in natural and experimental latency models of human cytomegalovirus and its correlation with histone modifications at a latent promoter. J. Gen. Virol 91, 599–604 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

