Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro
- PMID: 29584754
- PMCID: PMC5870973
- DOI: 10.1371/journal.pone.0194778
Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro
Abstract
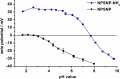
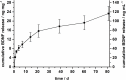
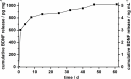
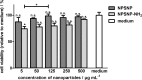
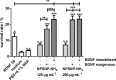
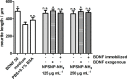
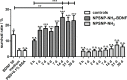
Sensorineural hearing loss (SNHL) can be overcome by electrical stimulation of spiral ganglion neurons (SGNs) via a cochlear implant (CI). Restricted CI performance results from the spatial gap between the SGNs and the electrode, but the efficacy of CI is also limited by the degeneration of SGNs as one consequence of SHNL. In the healthy cochlea, the survival of SGNs is assured by endogenous neurotrophic support. Several applications of exogenous neurotrophic supply have been shown to reduce SGN degeneration in vitro and in vivo. In the present study, nanoporous silica nanoparticles (NPSNPs), with an approximate diameter of <100 nm, were loaded with the brain-derived neurotrophic factor (BDNF) to test their efficacy as long-term delivery system for neurotrophins. The neurotrophic factor was released constantly from the NPSNPs over a release period of 80 days when the surface of the nanoparticles had been modified with amino groups. Cell culture investigations with NIH3T3 fibroblasts attest a good general cytocompatibility of the NPSNPs. In vitro experiments with SGNs indicate a significantly higher survival rate of SGNs in cell cultures that contained BDNF-loaded nanoparticles compared to the control culture with unloaded NPSNPs (p<0.001). Importantly, also the amounts of BDNF released up to a time period of 39 days increased the survival rate of SGNs. Thus, NPSNPs carrying BDNF are suitable for the treatment of inner ear disease and for the protection and the support of SGNs. Their nanoscale nature and the fact that a direct contact of the nanoparticles and the SGNs is not necessary for neuroprotective effects, should allow for the facile preparation of nanocomposites, e.g., with biocompatible polymers, to install coatings on implants for the realization of implant-based growth factor delivery systems.
Conflict of interest statement
Figures




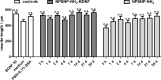
Similar articles
-
Stable release of BDNF from the fibroblast cell line NIH3T3 grown on silicone elastomers enhances survival of spiral ganglion cells in vitro and in vivo.Hear Res. 2012 Jul;289(1-2):86-97. doi: 10.1016/j.heares.2012.04.007. Epub 2012 Apr 28. Hear Res. 2012. PMID: 22564255
-
Promoting neurite outgrowth from spiral ganglion neuron explants using polypyrrole/BDNF-coated electrodes.J Biomed Mater Res A. 2009 Oct;91(1):241-50. doi: 10.1002/jbm.a.32228. J Biomed Mater Res A. 2009. PMID: 18814235
-
Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development.Neural Dev. 2011 Oct 11;6:33. doi: 10.1186/1749-8104-6-33. Neural Dev. 2011. PMID: 21989106 Free PMC article.
-
Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system.IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1138-48. doi: 10.1109/TBME.2007.895375. IEEE Trans Biomed Eng. 2007. PMID: 17551571 Free PMC article. Review.
-
Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors.Ann N Y Acad Sci. 1999 Nov 28;884:305-11. doi: 10.1111/j.1749-6632.1999.tb08650.x. Ann N Y Acad Sci. 1999. PMID: 10842602 Review.
Cited by
-
Neuro-nanotechnology: diagnostic and therapeutic nano-based strategies in applied neuroscience.Biomed Eng Online. 2023 Jan 2;22(1):1. doi: 10.1186/s12938-022-01062-y. Biomed Eng Online. 2023. PMID: 36593487 Free PMC article. Review.
-
Closing the Gap between the Auditory Nerve and Cochlear Implant Electrodes: Which Neurotrophin Cocktail Performs Best for Axonal Outgrowth and Is Electrical Stimulation Beneficial?Int J Mol Sci. 2023 Jan 19;24(3):2013. doi: 10.3390/ijms24032013. Int J Mol Sci. 2023. PMID: 36768339 Free PMC article.
-
Expression of neurotrophic factor genes by human adipose stem cells post-induction by deprenyl.Anat Cell Biol. 2021 Mar 31;54(1):74-82. doi: 10.5115/acb.19.229. Anat Cell Biol. 2021. PMID: 33526752 Free PMC article.
-
Molecular and Functional Characterization of BDNF-Overexpressing Human Retinal Pigment Epithelial Cells Established by Sleeping Beauty Transposon-Mediated Gene Transfer.Int J Mol Sci. 2022 Oct 26;23(21):12982. doi: 10.3390/ijms232112982. Int J Mol Sci. 2022. PMID: 36361771 Free PMC article.
-
Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders.Adv Drug Deliv Rev. 2019 Aug;148:239-251. doi: 10.1016/j.addr.2019.02.007. Epub 2019 Feb 21. Adv Drug Deliv Rev. 2019. PMID: 30797953 Free PMC article. Review.
References
-
- Wilson BS, Tucci DL, Merson MH, O´Donoghue GM. Global hearing health care: new findings and perspectives. Lancet 2017; 6736: 1–12. doi: 10.1016/S0140-6736(17)31073-5 - DOI - PubMed
-
- Li L, Chao T, Brant J, O’Malley B Jr, Tsourkas A, Li D. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv Drug Deliv Rev 2017; 108: 2–12. doi: 10.1016/j.addr.2016.01.004 - DOI - PMC - PubMed
-
- Kranz K, Warnecke A, Lenarz T, Durisin M, Scheper V. Phosphodiesterase Type 4 Inhibitor Rolipram Improves Survival of Spiral Ganglion Neurons In Vitro. PLoS One 2014; 9: e92157 doi: 10.1371/journal.pone.0092157 - DOI - PMC - PubMed
-
- Ramekers D, Versnel H, Strahl SB, Klis SFL, Grolman W. Temporary Neurotrophin Treatment Prevents Deafness-Induced Auditory Nerve Degeneration and Preserves Function. J Neurosci 2015; 35: 12331–12345. doi: 10.1523/JNEUROSCI.0096-15.2015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

