TRIM29 negatively controls antiviral immune response through targeting STING for degradation
- PMID: 29581886
- PMCID: PMC5859251
- DOI: 10.1038/s41421-018-0010-9
TRIM29 negatively controls antiviral immune response through targeting STING for degradation
Erratum in
-
Erratum: Author Correction to: TRIM29 negatively controls antiviral immune response through targeting STING for degradation.Cell Discov. 2018 May 31;4:25. doi: 10.1038/s41421-018-0031-4. eCollection 2018. Cell Discov. 2018. PMID: 29873323 Free PMC article.
Abstract
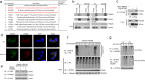
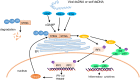
Innate immune system is armed by several lines of pattern recognition receptors to sense various viral infection and to initiate antiviral immune response. This process is under a tight control and the negative feedback induced by infection and/or inflammation is critical to maintain immune homoeostasis and to prevent autoimmune disorders, however, the molecular mechanism is not fully understood. Here we report TRIM29, a ubiquitin E3 ligase, functions as an inducible negative regulator of innate immune response triggered by DNA virus and cytosolic DNA. DNA virus and cytosolic DNA stimulation induce TRIM29 expression robustly in macrophages and dendritic cells, although the basal level of TRIM29 is undetectable in those cells. TRIM29 deficiency elevates IFN-I and proinflammatory cytokine production upon viral DNA and cytosolic dsDNA stimulation. Consistently, in vivo experiments show that TRIM29-deficient mice are more resistant to HSV-1 infection than WT controls, indicated by better survival rate and reduced viral load in organs. Mechanism studies suggest that STING-TBK1-IRF3 signaling pathway in TRIM29 KO cells is significantly enhanced and the degradation of STING is impaired. Furthermore, we identify that TRIM29 targets STING for K48 ubiquitination and degradation. This study reveals TRIM29 as a crucial negative regulator in immune response to DNA virus and cytosolic DNA, preventing potential damage caused by overcommitted immune responses.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
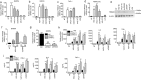



Similar articles
-
TRIM29 promotes DNA virus infections by inhibiting innate immune response.Nat Commun. 2017 Oct 16;8(1):945. doi: 10.1038/s41467-017-00101-w. Nat Commun. 2017. PMID: 29038422 Free PMC article.
-
TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING.PLoS Pathog. 2015 Jun 26;11(6):e1005012. doi: 10.1371/journal.ppat.1005012. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26114947 Free PMC article.
-
TRIM29 Negatively Regulates the Type I IFN Production in Response to RNA Virus.J Immunol. 2018 Jul 1;201(1):183-192. doi: 10.4049/jimmunol.1701569. Epub 2018 May 16. J Immunol. 2018. PMID: 29769269 Free PMC article.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
TRIM29 in Cutaneous Squamous Cell Carcinoma.Front Med (Lausanne). 2021 Dec 20;8:804166. doi: 10.3389/fmed.2021.804166. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34988104 Free PMC article. Review.
Cited by
-
Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity.PLoS Pathog. 2022 May 18;18(5):e1010544. doi: 10.1371/journal.ppat.1010544. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35584187 Free PMC article.
-
Modulation of Ubiquitin Signaling in Innate Immune Response by Herpesviruses.Int J Mol Sci. 2022 Jan 1;23(1):492. doi: 10.3390/ijms23010492. Int J Mol Sci. 2022. PMID: 35008917 Free PMC article. Review.
-
TRIM Expression in HNSCC: Exploring the Link Between Ubiquitination, Immune Infiltration, and Signaling Pathways Through Bioinformatics.Int J Gen Med. 2024 May 24;17:2389-2405. doi: 10.2147/IJGM.S463286. eCollection 2024. Int J Gen Med. 2024. PMID: 38808201 Free PMC article.
-
MITA oligomerization upon viral infection is dependent on its N-glycosylation mediated by DDOST.PLoS Pathog. 2022 Nov 30;18(11):e1010989. doi: 10.1371/journal.ppat.1010989. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36449507 Free PMC article.
-
Current understanding of the cGAS-STING signaling pathway: Structure, regulatory mechanisms, and related diseases.Zool Res. 2023 Jan 18;44(1):183-218. doi: 10.24272/j.issn.2095-8137.2022.464. Zool Res. 2023. PMID: 36579404 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

