Leydig cells: formation, function, and regulation
- PMID: 29566165
- PMCID: PMC6044347
- DOI: 10.1093/biolre/ioy059
Leydig cells: formation, function, and regulation
Abstract
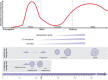
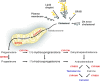
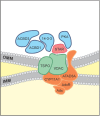
Herein we summarize important discoveries made over many years about Leydig cell function and regulation. Fetal Leydig cells produce the high levels of androgen (testosterone or androstenedione, depending upon the species) required for differentiation of male genitalia and brain masculinization. Androgen production declines with loss of these cells, reaching a nadir at postpartum. Testosterone then gradually increases to high levels with adult Leydig cell development from stem cells. In the adult, luteinizing hormone (LH) binding to Leydig cell LH receptors stimulates cAMP production, increasing the rate of cholesterol translocation into the mitochondria. Cholesterol is metabolized to pregnenolone by the CYP11A1 enzyme at the inner mitochondrial membrane, and pregnenolone to testosterone by mitochondria and smooth endoplasmic reticulum enzymes. Cholesterol translocation to the inner mitochondrial membrane is mediated by a protein complex formed at mitochondrial contact sites that consists of the cholesterol binding translocator protein, voltage dependent anion channel, and other mitochondrial and cytosolic proteins. Steroidogenic acute regulatory protein acts at this complex to enhance cholesterol movement across the membranes and thus increase testosterone formation. The 14-3-3γ and ε adaptor proteins serve as negative regulators of steroidogenesis, controlling the maximal amount of steroid formed. Decline in testosterone production occurs in many aging and young men, resulting in metabolic and quality-of-life changes. Testosterone replacement therapy is widely used to elevate serum testosterone levels in hypogonadal men. With knowledge gained of the mechanisms involved in testosterone formation, it is also conceivable to use pharmacological means to increase serum testosterone by Leydig cell stimulation.
Figures
Similar articles
-
Leukemia inhibitory factor antagonizes gonadotropin induced-testosterone synthesis in cultured porcine leydig cells: sites of action.Endocrinology. 2001 Jun;142(6):2509-20. doi: 10.1210/endo.142.6.8177. Endocrinology. 2001. PMID: 11356700
-
Restoration effects of exogenous luteinizing hormone on the testicular steroidogenesis and Leydig cell ultrastructure.Endocrinology. 1985 Nov;117(5):1779-87. doi: 10.1210/endo-117-5-1779. Endocrinology. 1985. PMID: 4042963
-
Influences of flavones on cell viability and cAMP-dependent steroidogenic gene regulation in MA-10 Leydig cells.Cell Biol Toxicol. 2018 Feb;34(1):23-38. doi: 10.1007/s10565-017-9395-8. Epub 2017 Apr 28. Cell Biol Toxicol. 2018. PMID: 28455626
-
Testosterone recovery therapy targeting dysfunctional Leydig cells.Andrology. 2023 Jul;11(5):816-825. doi: 10.1111/andr.13304. Epub 2022 Oct 10. Andrology. 2023. PMID: 36164998 Review.
-
Regulation of Leydig cell steroidogenic function during aging.Biol Reprod. 2000 Oct;63(4):977-81. doi: 10.1095/biolreprod63.4.977. Biol Reprod. 2000. PMID: 10993816 Review.
Cited by
-
The cost of the circadian desynchrony on the Leydig cell function.Sci Rep. 2022 Sep 15;12(1):15520. doi: 10.1038/s41598-022-19889-9. Sci Rep. 2022. PMID: 36109553 Free PMC article.
-
A Dietary Supplement Jinghuosu Ameliorates Reproductive Damage Induced by Tripterygium Glycosides.Chin J Integr Med. 2024 Apr;30(4):330-338. doi: 10.1007/s11655-023-3750-9. Epub 2024 Jan 12. Chin J Integr Med. 2024. PMID: 38212501
-
Heat stress and stallion fertility.J Anim Sci Technol. 2023 Jul;65(4):683-697. doi: 10.5187/jast.2023.e29. Epub 2023 Jul 30. J Anim Sci Technol. 2023. PMID: 37970501 Free PMC article. Review.
-
Taste receptor type 1 member 3 is required for the fertility of male mice.Heliyon. 2024 Jan 18;10(2):e24577. doi: 10.1016/j.heliyon.2024.e24577. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38312691 Free PMC article.
-
Testicular pathological alterations associated with SARS-CoV-2 infection.Front Reprod Health. 2023 Jun 29;5:1229622. doi: 10.3389/frph.2023.1229622. eCollection 2023. Front Reprod Health. 2023. PMID: 37457430 Free PMC article. Review.
References
-
- Leydig F. Zur Anatomie der männlichen Geschlechtsorgane und Analdrüsen der Säugethiere. Zeitschrift f Wiss Zool 1850;2:1–57.
-
- Le Minor JM, Sick H. About the 350th anniversary of the foundation of the chair of anatomy of the faculty of medicine at Strasbourg (1652-2002). Hist Sci Med 2003;37:31–40. - PubMed
-
- Bouin P, Ancel P. Recherches sur les cellules interstitielles du testicle des mammifères. Arch Zool Exp Gen 1903;1:437–523.
-
- Hall PF, Eik-Nes KB. The action of gonadotropic hormones upon rabbit testis in vitro. Biochim Biophys Acta 1962;63(3):411–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

